zeppelin69
Harmless

Posts: 46
Registered: 20-2-2007
Member Is Offline
Mood: No Mood
|
|
Compatibility of explosives.
I know this question will probably raise a few eyebrows, most of you will probably think if I don't know the answer to it. Then I shouldn’t be
fooling with the compounds anyway, but I was wondering if anyone could advise me whether or not it is an extremely bad idea to mix trinitrophenol and
nitroglycerin. Both will be in small quantities and it will be disposed of quickly, but I am wondering if there will be an issue with the TNP being an
acid and making the NG even more unstable than it already is.
I hope this isn't too kewlish, I was simply trying to work out a better oxygen balance for TNP. I would appreciate it if anyone with any idea (or even
better some experience) could enlighten on how bad/good of an idea this is. Thanks.
|
|
|
Sickman
Hazard to Self
 
Posts: 98
Registered: 9-5-2004
Member Is Offline
Mood: Icy and I see!
|
|
zeppelin69,
Adding nitroglycerin to TNP( Picric Acid) is not advisable, nor is it neccesary!
TNP is an outstanding explosive in and of itself, and in my opinion needs no improvement. When properly pressed or cast TNP puts out a brisant
impulse; the shock wave resulting from the high order detonation of TNP can cut any metal on your periodic table or anything else for that matter, in
my opinion.
If you want or need more bang, just use more TNP. If your still determined to make every microscopic improvement in performance that is possible then
you can just add a little powdered aluminum and/or a little sodium nitrate. All in all I'd just say to use a little more TNP for whatever. Since
explosives are for the overwhelming majority of uses, used to destroy, a little extra TNP doesn't hurt and if it does, O well, it's supposed to! 
|
|
|
vulture
Forum Gatekeeper
    
Posts: 3330
Registered: 25-5-2002
Location: France
Member Is Offline
Mood: No Mood
|
|
So you're going to add aluminium powder to acidic TNP?
You can always use the sodium salt of TNP.
One shouldn't accept or resort to the mutilation of science to appease the mentally impaired.
|
|
|
Sickman
Hazard to Self
 
Posts: 98
Registered: 9-5-2004
Member Is Offline
Mood: Icy and I see!
|
|
Firstly, dry TNP doesn't form aluminum picrate with dry aluminum powder and nor does it form in an aluminum blasting cap housing when the contents are
kept dry and as Rosco Bodine has already pointed out Picric Acid is NOT hygroscopic when pure.
Secondly, sodium nitrate also will not react in dry conditions with picric acid. Besides even if the sodium picrate monohydrate were to form it is a
safer explosive than TNP and presents no additional hazard.
Thirdly, I still maintain that picric acid needs no improvement whatsoever. If more performance is required the simple use of MORE picric acid is
called for. End of story!
|
|
|
chemrox
International Hazard
    
Posts: 2961
Registered: 18-1-2007
Location: UTM
Member Is Offline
Mood: LaGrangian
|
|
why mess with so unstable a compound as nitroglycerine? Picric is a little dicey when dry and pure too but a lot easier to work with than
nitroglycerine. The most hazardous occupation during the Civil War was being a nitro chemist. They has them at benches in big barnlike structures
with wide doorways that had ramps. Each chemist had a bell next to his bench and when he saw his thermometer start going up too fast he hit the bell
and dove out the door.
"When you let the dumbasses vote you end up with populism followed by autocracy and getting back is a bitch." Plato (sort of)
|
|
|
vulture
Forum Gatekeeper
    
Posts: 3330
Registered: 25-5-2002
Location: France
Member Is Offline
Mood: No Mood
|
|
| Quote: |
Firstly, dry TNP doesn't form aluminum picrate with dry aluminum powder |
Well ofcourse. Neither does dry citric acid react with dry sodiumcarbonate. But which homemade TNP is really dry?
| Quote: |
Besides even if the sodium picrate monohydrate were to form it is a safer explosive than TNP and presents no additional hazard.
|
And that is precisely why I mentioned. Its merit is twofold. It's more stable than TNP and most importantly, it isn't acidic.
| Quote: |
Thirdly, I still maintain that picric acid needs no improvement whatsoever. If more performance is required the simple use of MORE picric acid is
called for. End of story! |
Amen to that.
One shouldn't accept or resort to the mutilation of science to appease the mentally impaired.
|
|
|
Boomer
Hazard to Others
  
Posts: 190
Registered: 11-11-2005
Member Is Offline
Mood: No Mood
|
|
On a side note, while both Al powder and sodium nitrate enhance total energy, they both also lower brisance (or CJ pressure for that matter). Al does
so even more than an inert diluent like table salt would. Axt attached an article about this somewhere.
If you need more brisance (not more power!), use a more brisant explosive. 190 kilobar on twice the area is NOT the same as 380 kilobar.
But I agree that you hardly need that, e.g. unless you already use molybdenum liners, charge precision will have more impact on SC performance than
explosive choice.
Also, I remember it's not the same to have badly washed nitro, than to have nitro plus an acid like TNP. Just think ammonia (extra) dynamites. While
AN is acidic, they store well. And it's not because they contain enough anti-acid to neutralise all AN.
Oh and if you wanna improve TNP's OB, dissolve in fuming NA! 
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
Acidic compounds are miscible in each other and an avenue for investigation of
blended formulations. Rosco Bodine discussed Picric acid with Nitromethane here _
http://www.sciencemadness.org/talk/viewthread.php?tid=6785&a...
We know nitromethane is sensitized by mixing in small amounts of basic amine
compounds. I have wondered also at the stabilizing effect nitromethane might
have on Urea nitrate and nitro and Dinitro ureas which degrade so rapidly as to
be unsuitable for storage. An interesting result of this combination is enhanced
oxygen balance. Nitromethane has an oxygen deficit , nitro ureas have oxygen
in excess. Equimolar amounts assure combustion of all the hydrogen and carbon
present to the monoxide. Approximate weight ratios for proposed mixtures are
shown in brackets [ ]
[ 11 : 4 ] , CO(NH2)2.HNO3 + CH3NO2 -> 2 CO + 4 H2O + 2 N2
[ 7 : 4 ] , CO(NH2)HNNO2 + CH3NO2 -> 2 CO + 3 H2O + 2 N2
Dinitrourea actually results in complete combustion to CO2
[ 10 : 4 ] , 4 CO(HNNO2)2 + 4 CH3NO2 -> 8 CO2 + 10 H2O + 10 N2 + O2
.
|
|
|
Sickman
Hazard to Self
 
Posts: 98
Registered: 9-5-2004
Member Is Offline
Mood: Icy and I see!
|
|
| Quote: | Originally posted by franklyn
I have wondered also at the stabilizing effect nitromethane might
have on Urea nitrate and nitro and Dinitro ureas which degrade so rapidly as to
be unsuitable for storage. |
Franklyn,
If urea nitrate is so unstable in storage then why is urea used to stabilize nitric esters?
On an unrelated note: thank you for your input and suggestions for further study on primary explosives!
Sickman
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
Urea nitrate does keep better than it's nitroamine derivatives.
Urea alone is not the same as it's nitrate whatever its added to.
.
|
|
|
Sickman
Hazard to Self
 
Posts: 98
Registered: 9-5-2004
Member Is Offline
Mood: Icy and I see!
|
|
| Quote: | Originally posted by franklyn
Urea alone is not the same as it's nitrate whatever its added to.
. |
Doesn't urea form urea nitrate from the nitric ester it's added to as the nitric ester loses some of it's nitrogen content in the form of nitrous and
nitric acids which promote a further decomposition? That is what I am implying! Or is this not the path of reaction that occurs upon decompostion of a
nitric ester in presents of urea as a stabilizer?
I tend to think that as the nitric ester continues to slowly give off it's assumed products of decomposition in storage that the urea would continue
to achieve higher and higher levels of nitration untill either the waste products of the decomposition of the nitric ester is exausted or the urea's
nitration is completed to the theoretical maximum before it itself decomposes, further adding to the decomposition of the nitric ester which it was
intended to stabilize.
|
|
|
Mario.
Harmless

Posts: 7
Registered: 13-1-2008
Location: Depends when.
Member Is Offline
Mood: disoriented
|
|
Glyceroltrinitrate stability
There are two isomers of glyceroltrinitrate.. one is rather stable and useable, the other is extremely sensitive and useless, they are cis- and trans-
modifications. As far as I remember, the only difference is that one has all three -O-NO2 groups in line and the other has the middle one turned
upside. The melting temperature of one and the other is radically different. For exact temperatures, etc see general small dictionary of chemistry.
I vaguely remember that pure form of one of the isomers can not stand even slightest shock, nor it can be heated to body temperatures without
detonating. I have see the other form on youtube (swedish?) to be rather stable, sometimes not even going off when hit by a hammer on aluminium?
There used to be fuels based on nitroglycerine for turbines, but just experimental. "nitromethane" is still used as a special racing fuel, it is just
a bit different from the nitroglycerine.
Plus, I needed only two things to know about picric acid:
1) it is sensitive 2) vapours form picrates with metals (in production of picric acid, wooden ventilation was common.)
3) used in production of grenades. (plus in alloy with TNT or something...)
If I had to choose, I would rather handle the NG and soak it into absorbent, rather than handling crystalline matter that tends to go off in
uncontrolled environments and on static discharge (impurities greatly increase sensitivity, so do salts)
Glad to find a place with fellow mad scientists... oops! Mad science is forbidden under the antiterrorist act. Let's talk about Pasteurization of milk
instead!
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
@ Sickman
That's quite a confused farrago of notions. First, please proof read your post
beforehand as inadvertent spelling errors confuse the meaning of what you write.
I had previously made an error in naming these class of compounds, R-NR'-NO2
http://www.sciencemadness.org/talk/viewthread.php?tid=6042&a...
which not_important corrected me in this post above, these are NOT
called Esters, they are AMIDES. By ester I am assuming you mean Methyl,
Ethyl, Ethylene Glycol, or Glycerol, nitrates. I take it on faith that adding urea
to an ester is practiced as this is the first I hear of this. Anyway urea would not
be to " stabilize " it, as the alkyl esters I just mentioned are chemically fairly
unreactive. The only reason I can see to add a small bit of urea might be to
neutralize whatever residual acidity may be present from their nitration since
acidity promotes the formation of bubbles on storage and particularly liquid
explosives such as alkyl esters become sensitized by this. Issues of sensitivity
are another matter and Nobels dynamite formulation of absorbing the liquid ester
into diatomaceous earth, now called zeolites, adequately deals with that. Finally,
when blasting, urea may be included as an additive in the explosive material to
reduce the temperature of the explosion, it will not combine chemically with the
other compounds present except to neutralize remnant free mineral acid.
Nitroamides of the form R-NH-NO2 such as Ureas or MEDINA are inherently acid
and as such unavoidably become hydrolysed rather quickly, unless kept ultra dry.
( Even Keto RDX, which is not acid, having a structural similarity, is prone to
degradation of this type ) Combining with a basic Amine compound such as urea,
guanidine, or alkyl amines, produces a pH neutral adduct which is less prone to
degradation but also lowers the explosives performance. I posted on the same
speculative notion here myself.
http://www.sciencemadness.org/talk/viewthread.php?tid=6042&a...
.
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
Dinitrourea + Nitroguanidine , Adduct
From the book Numerical Modeling of Explosives & Propellants C.L.Mader
Some jargon deleted by me for clarity , my additions appear inside { brackets }
Pg 61
The effect of adding " exotic " elements such as Boron , Aluminum , or Fluorine
has been investigated because the heat of detonation is as much as doubled.
Usually the observed pressures and detonation velocities are not as high as those
of the better CHNO explosives at the same densities. The poor performance of the
Boron and Fluorine explosives relative to CHNO explosives results from formation of
complex detonation product molecules such as B2О3 and BF3.
Pg 63
The most important contribution to our understanding of the detonation process
has been the discovery that the performance of an explosive is a very sensitive
function of the { number } of detonation products. Systems with high heats of
explosion yield detonation products that have large molecular weights and hence
low specific { number } of particles , so the extra energy is present primarily as
thermal energy rather than intermolecular { repulsion }.
A promising approach has been to look for higher density CHNO explosives with
compositions similar to HMX. TATB is one explosive whose high density makes its
performance 30% greater than that of TNT even though its composition and heat
of detonation are similar. { Detonation products per mol TNT 1 : 11 , two are Carbon ,
for TATB 1 : 12 , no Carbon } Nitroguanidine has a heat of detonation half that of
Composition B , but it has the same performance because of the favorable particle
population of detonation products resulting from the high hydrogen content in the
explosive and consequent water content in the detonation products.
(1) Opportunity for improvement on existing explosives is not limited to creation
of new compounds. Many materials dismissed and neglected due to deficiency
have unrealized potential. Exploitation of overlooked properties can remedy
undesirable characteristics. The following excerpts outline concerns with two of
the best known , Nitroguanidine and Dinitrourea , which I will argue is remediable
by using both combined.
_______________________________________________________________
Organic Chemistry of Explosives J.P. Agrawal , R.D. Hodgson
From page 194 _
The facile hydrolysis of secondary nitramides to primary nitramines in the presence
of aqueous acid or base and, in some cases, in prolonged contact with hot water,
has undoubtedly limited their use as practical explosives.
N -Nitroureas are an interesting group of compounds. The simplest member, Nitrourea
(NU), is a labile substance and readily decomposes in the presence of water.
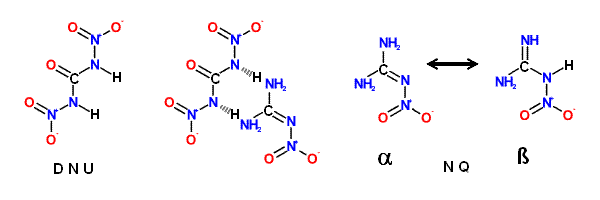
N, N' -Dinitrourea (DNU), although a powerful explosive, shows similar properties.
N -Substituted-N-nitroureas are more hydrolytically stable. Much interest has
focused on the incorporation of the N-nitrourea functionality into cyclic and caged
structures because of the increase in performance observed. This is due to
increased crystal density, which is attributed to the rigidity of the urea functionality.
The N-substituted N-nitrourea functionality is also associated with a low sensitivity
to impact a property possibly due to intramolecular hydrogen bonding in the
nitrourea framework. One such compound, 1,4-dinitrog]ycouril (DINGU), is classified
an insensitive high explosive (IHE) and exhibits good performance (VOD ~ 7580 m/s ,
density = 1.99 g/cc). N.N-Disubstituted-N,N'-dinitroureas are also associated with
high performance. However, many cyclic N,N'-dinitroureas are hydrolytically unstable
and decompose on contact with water. Such compounds will never find use as
practical explosives.
Some compounds can be drawn as primary nitramines or as nitrimines. The two
groups are tautomeric but have very different properties. Nitrimines do not contain
acidic hydrogen and so their solutions are neutral. Nitroguanidine exists in the
nitrimine form under norrnal conditions, and although its structure can be drawn as
a primary nitramine, its properties are not consistent with such a structure.
Nitroguanidine is a compound of some importance in the explosives industry as a
component of triple-base propellants and also as a precursor to other explosives.
The low combustion temperature of nitroguanidine containing gun propellants makes
thern both flash!ess and less erosive to gun barrels, a consequence of the high
nitrogen content of nitroguanidine (CH4N402 = 54% N), Although nitroguanidine is
an explosive, its fibrous nature imparts an extremely low density to the compound
even on compression, and consequently, it exhibits low performance; this factor
alone limits the use of nitroguanidine as an explosive. If nitroguanidine were to exist
as a primary nitramine under normal conditions it is unlikely it would have found wide
applications in explosive technologies.
_ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _
Explosive Effects and Applications , Jonas A. Zukas , William P. Walters
From page 157
Nitroguanidine ( NQ ) is an exception to the rule that most primary nitramines
have no practical application as explosives. It forms low melting eutectics with
ammonium nitrate ( AN ) and Guanidine Nitrate ( GN ).
20% NQ + 80% AN , 132ºC
41% NQ + 59% GN , 167ºC
17.5% NQ + 22.5% GN + 60% AN , 113ºC
_ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _
Nitroguariidine ( NQ ), a guanyl nitramine or nitramino compound, is a colorless
crystalline solid that exists in two tautomeric forms. The ' α ' form; usually produced
during manufacture of NQ, consists of long, thin, flat needles that are tough and
difficult to pulverize; this form predominates in acidic; neutral; or slightly basic media.
The ' α ' form crystallizes from water in clusters of small thin, elongated plates.
The explosive power of NQ is approximately 77 % that of trinitrotoluene (TNT), although
at a density of 1.55 g/cc it has a higher rate of detonation ( 7,650 m/sec ) than TNT
has at the same density ( 6,900 rn/sec ). NQ begins to undergo decomposition at a
higher temperature ( 232°C ) than does TNT ( 80 to 200°C ); both are essentially
nonhydroscopic, on the same order of stability and soluble in water,
Heat Of combustion = 209 kcal / mol
Derisity = 1.72 g/cc
Stability characteristics = ' α ' is stable form
Physical state = Colorless crystalline solid; exists in two forms :
α, long thin flexible, lustrous needles ; β and small, thin, elongated plates
Solubility characteristics α form
Water : 4.4 g /L at 25ºC , 82.5 g /L at 100ºC
Basic : ( in KOH ) 12 g /L at 25ºC
Acid : (40% H2SO4) 80 g /L at 25ºC
Acetonitrile : Soluble
Alcholhol : Slight solubility
Ether : Insoluble
_ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _
Acidity of Nitroguanidine and it's Homologues
Can. J. Cllem. Vol. 39 , pp 1787-1796 (1961)
http://article.pubs.nrc-cnrc.gc.ca/ppv/RPViewDoc?_handler_=HandleInitialGet&journal=cjc&volume=39&calyLang=fra&articleFile=v61-235.pdf
" It is now recognized that Nitroguanidine exists commonly as the Nitrimine,
H2N.C(NN02).NH2 , rather than as the tautomeric primary Nitramine."
( * My note : Nitramine NH2.C(NH).NHNO2 type forms salts with strong alkali )
" The basicity of Nitroguanidine has been reported as pKb = 14.5 (10). Indeed the
existence of salts such as the hydrochloride (11) in media minimal in content of
water , would indicate that the basicity is comparable with that of Urea
( Kb, at 25ºC , 1.5 X 10ˉ14 )" ( * My note : urea pKa ~ 26.9
we see NQ's characteristic proprties shows it to be amphoteric )
10. L. H. Hall , J. E. De Vries , and E.S. Gantz. J. Am. Chem. Soc. 77, 6507 (1955).
11. J. Thiele. Ann. 270, 1 (1892).
_ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _
Nitroguanidines A. F. McKAY , page 305
Jousselin (58) first observed that nitroguanidine crystallized from warm hydrochloric
acid or nitric acid solution on cooling as the hydrochloride or nitrate salt, respectively.
These salts were readily hydrolyzed by water, indicating that nitroguanidine has only
weakly basic properties. Wood (137) determined the extent of dissociation of
nitroguanidine hydrochloride in N/25 solution by its effect on the rate of hydrolysis
of methyl acetate. This method showed that a N/25 solution of nitroguanidine
hydrochloride is dissociated to the extent of 97.5 per cent. A 94 per cent dissociation
was calculated for a N/10 solution and the dissociation constant of the base at 40.2 ºC.
was calculated to be 2.1 X 10ˉ14 This is just slightly less than the value for urea
(3.7 X 10ˉ14 ) or acetamide (3.3 X 10ˉ14 ). The slight basicity of nitroguanidine
is responsible for its increased solubility in acid solutions.
(58) Jousselin, L.: Compt. rend. 88, 814 (1879).
(137) Wood, J.K.: J. Chem. Soc. 83, 568 (1903).
_ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _
Structure of Nitroguanidine and of it's Derivatives
J. Org. Chem. 18 (6), pp 629–642 (1953)
http://pubs.acs.org/doi/abs/10.1021/jo01134a003
" Evidence is presented to show that Nitroguanidine and many of it's derivatives
exit in the Nitrimino rather than the Nitramino form , although conversion to the
latter tautomer may occur in alkaline solution."
_ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _
Two Forms of Nitroguanidine , J. Am. Chem. Soc. 47 , 1063 (1925)
T.L. Davis , A.A. Ashdown , H.R. Couch
" Neither substance appears to be an aci form or a stronger pseudo acid than the
other, for they have essentially the same solubility in 0.1 N Potassium Hydroxide
solution. Neither is altered by recrystallization from Glacial Acetic acid or from
Ammonia water. They yield identical nitrates and hydrochlorides, and these salts
on recrystallization from water yield only α-Nitroguanidine."
" Salts of Nitroguanidine : When either form of nitroguanidine is dissolved in hot,
conc. nitric acid and allowed to crystallize, apparently identical nitrates are
deposited in thick rhombic shaped prisms ; m.p. 147ºC , with decomposition.
They lose Nitric Acid slowly in the air and yield α-Nitroguanidine when recrystallized
from water.
By recrystallization of either form from conc. Hydrochloric Acid, apparently
identical hydrochlorides are obtained, crystallizing in needles. The crystals lose
Hydrogen Chloride rapidly in the air, and show the melting point of Nitroguanidine.
If recrystallized from water, they yield the alpha compound."
" They are distinguished by their indices of refraction, and differ greatly
in crystal habit and slightly in solubility in water.
The beta form is converted to the alpha form by the action of strong
mineral acids; otherwise there appears to be no chemical difference between
the two, for they show the same reactions and yield identical derivatives."
_ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _
Military Explosives , TM 9-1300-214 , page 8-42 to 8-46
www.freepyroinfo.com/Pyrotechnic/Pyrotechnic_Books/Tm_9_1300...
page 8-43 _
" Nitroguanidine is chemically reactive because of a free amino group as well as
a nitro group and a tendency toward dearrangement. With hot concentrated
nitric acid, nitroguanidine forms a nitrate that melts at 147 °C and, with strong
hydrochloric acid, yields a crystalline hydrochloride."
" nitroguanidine is relatively stable in aqueous solution. A saturated
aqueous solution at 25°C has a pH value of 5.5.
_______________________________________________________________
(2) Dinitrourea has high density , excess oxygen balance , and being
tautomeric , is acid in character , eventually deteriorating unless it's hydrogen
is bound to a base. ( See references and attachment below )
In view of the observed properties of Nitroguanidine , low density , low oxygen
balance , but more importantly ability to act as an organic base , it appears that
complimentary properties of Dinitrourea can remediate the shortcomings of both
if Nitroguanidine can be crystallized as an adduct with Dinitrourea. Projected
performance even at the nominal density of ρ 1.88 shows considerable
improvement despite zero oxygen balance reducing combined gas particle count
from ' 5 ' for NQ and ' 5 ' for DNU , to ' 9 ' for a 1 : 1 molar composition.
NQ - (NH2)2CNNO2 => CO + H2O + 2 N2 + H2
DNU - (HNNO2)2CO => CO2 + H2O + 2 N2 + O2
(NH2)2CNNO2 • (HNNO2)2CO => 2 CO2 + 3 H2O + 4 N2
Projections of performance vary depending on methodology , it is
notworthy that some estimates are comparable to Keto-RDX.
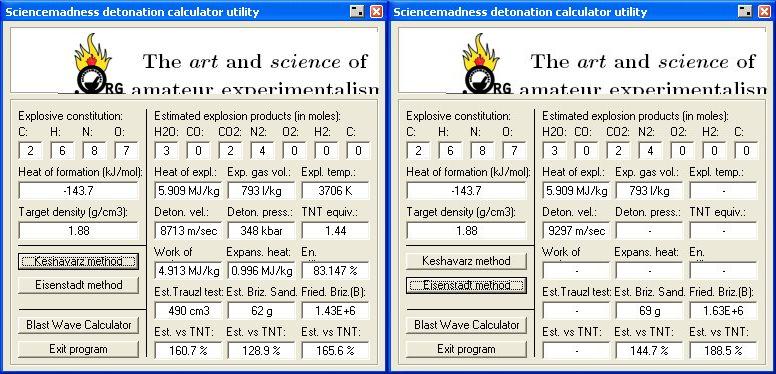
Result 's using Engager 's Detonation utility
http://www.sciencemadness.org/talk/files.php?pid=169218&...
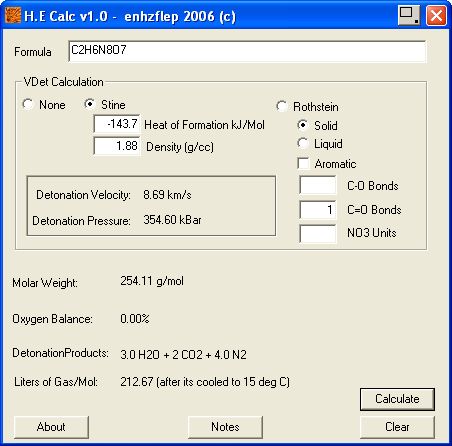
and enhzflep 's
High Explosives Calculator v 1.0 , are attached below.
http://www.sciencemadness.org/talk/files.php?pid=64852&a...
To estimate the Detonation pressure generated and Velocity of Detonation ,
I have applied the method of M.J. Kamlett, S.J. Jacobs outlined in NOL TR 67 - 66
http://handle.dtic.mil/100.2/AD661483 , and also The Journal of Chemical Physics , vol 48 , num 1 ,
A Simple method for Calculating Detonation Properties of C-H-N-O Explosives
discussed at length here _
http://www.sciencemadness.org/talk/viewthread.php?tid=11195&...
Corrected calculation for in a later post here
http://www.sciencemadness.org/talk/viewthread.php?tid=11195&...
_ _ _ _ _ _ _ _ _ _ _ _ _
Density is ρ is simply estimated by averaging the measured
values of both substances. Density of Dinitrourea is 1.98 gm/cm³
Density of Niroguanidine is not so clear , nominal densities have
been observed from 1.55 up to 1.77 the LANL established practical limit.
The median value of NQ & DNU as a compound is 1.88 gm/cm³
Since even organic salts ordinarilly achieve higher density than either of the
acid or base components alone , it is very possible this composition could
exceed the density value of Dinitrourea 1.98 gm/cm³
_ _ _ _ _ _ _ _ _ _ _ _ _
∆Hf Heat of formation values in kilojoules per mol
are - 58.8 for DNU , See attached zip file
and - 84.9 for NQ , , based on NQ ~ 20.29 kcal/mol , Military Explosives Page 8-42
∆Hf Heat of formation without regard to adduct enthalpy of DNU / NQ
(- 58.8 , DNU) + (- 84.9 , NQ) = - 143.7 . KJ/mol
The following ∆Hf (Heat of formation) values in kilocalories per mol
are applied for thermodynamic calculations (- 143.7 . KJ/mol ) / 4.186 =
- 34.3 Kcal /mol
- 57.8 for H2O ( g ), - 94 for CO2
∆Hf Heat of formation of the NQ / DNU adduct is subtracted from the ∆Hf Heat of formation
of detonation products to infer the Heat of explosion: (NH2)2CNNO2 • (HNNO2)2CO => 2 CO2 + 3 H2O + 4 N2
(2 CO2 is - 188) + (3 H2O ( g ) is - 173.4) + (4 N2 is zero) - (- 34.3 ) = - 327.1 kcal /mol
Dividing the ∆He Heat of explosion of 1 mol of the compound by it's molar weight , - 327.1 / 254.15
obtains Heat of explosion ∆He = 1 2 8 7 Kcal per kilogram of compound
( The modest energy product results from the small carbon content )
M is grams of gas per mol of gas , equation 14 page 13 , in NOL TR 67 - 66
N is mols of gas per gram , equation 13 page 13 , in NOL TR 67 - 66
P is detonation pressure expressed in kilobars ( 1 Bar = 1 atmosphere )
ρ density given as grams per cubic centimeter 1.88
Q is ∆He Heat of explosion in calories per gram 1 2 8 7
So this can be easily understood ,
the constants that count each type of atom , given in the article as a , b , c , d
in the formulas for M and N , are changed here to the atom each represents a = C (Carbon)
b = H (Hydrogen) , c = N (Nitrogen) , d = O (Oxygen)
M = . . .56 N + 88 O - 8 H . . . = . . . 56( 8 ) + 88( 7 ) - 8( 6 ) . . . = 28.222 . .
. . . . . . . . 2 N + 2 O + H . . . . . . . . . . 2( 8 ) + 2( 7 ) + ( 6 )
N = ... 2 N + 2 O + H . . . . . . . . . . = . . . .2( 8 ) + 2( 7 ) + ( 6 ) . . . . . . . . . . . . . .≈ 0.035433
. . . . 48 C + 4 H + 56 N + 64 O . . . . . . 48( 2 ) + 4( 6 ) + 56( 8 ) + 64( 7
)
The formulas given for M and N intend to acount
for what carbon remains unreacted
in explosives with negative oxygen balance. This does not apply in this case and the
value of M X N = G = calculates to 1.0 , indicating a corrective subtraction of - 6 %
We now have all the necessary values to calculate this : ( N √ M √ Q )
( Q ( 1 2 8 7 ) is substituteded here without
regard for sign )
( * Note this expression is given as ф ( phi ) in the article ) . . . ( 0.0354 ( 5.31 ) ( 35.87 ) ) = 6.75
Detonation pressure = P = 15.58 ρ ² ( N
√ M √ Q )
. . . . . . . . . . . . . . . . . . . . . . . . . 15.58 ( 1.88 ) ² ( 6.75 ) = 3 7 2
kilobars
minus - 24 , 6% correction factor comes to 3 4 9 kilobars
See ( 16 ) page 22 , in NOL TR 67 - 66
This would be ~ 396 kilobars at a density of 2
gm/cm³
That is comparable to HMX in performance.
Velocity of Detonation = VOD = 1.01 ( 1 + 1.3 ρ
) √ ( N √M √Q)
. . . . . . . . . . . . . . . . .1.01 ( 1 + 1.3 ( 1.88 ) ) √ ( 6.75 ) = 9.030 millimeters per microsecond
multiplied by one million to obtain meters per second ,
comes to ≈ 9 0 3 7 Meters / sec
9 4 5 4 Meters / sec at a density of 2 gm/cm³
_ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _
_ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _
Given the relative ease with which NQ & DNU are individually made , the projected
performance increase of their use combined as an adduct is a prospect well worth
investigating.
DNU posts
http://www.sciencemadness.org/talk/viewthread.php?tid=6042
Axt on Nitroguanidine
http://www.sciencemadness.org/talk/viewthread.php?tid=8911
Engager on Nitroguanidine
http://www.sciencemadness.org/talk/viewthread.php?tid=12938
Other references _
Impact Insensitive Dianionic Dinitrourea Salts
http://pubs.acs.org/doi/abs/10.1021/ef900691q
The zip file contains an html copy of the google cache of this article
Dense energetic salts of N, N" -dinitrourea
http://www.rsc.org/publishing/journals/NJ/article.asp?doi=b7...
.
Attachment: Dense energetic salts of N,N-dinitrourea (DNU).html.zip (233kB)
This file has been downloaded 686 times
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
This makes it conclusive, if Nitroguanidine forms salt adducts with
mineral acids HCl and HNO3 , it can certainly do so with Dinitrourea.
Crystal Structures and EPR Spectra of Nitroguanidine Chloride and Nitroguanidine Nitrate
http://www.informaworld.com/smpp/ftinterface~db=all~content=...
.
|
|
|
quicksilver
International Hazard
    
Posts: 1820
Registered: 7-9-2005
Location: Inches from the keyboard....
Member Is Offline
Mood: ~-=SWINGS=-~
|
|
@ franklyn :
That was an excellent summery and a wealth of information that some of it I didn't know. I had an interest in nitoguanadine & that was a quite
welcome addition.
Thank you for taking the time to make such a well outlined post: that must have taken a bit of doing.
[Edited on 14-1-2011 by quicksilver]
|
|
|
VladimirLem
Hazard to Others
  
Posts: 204
Registered: 24-5-2010
Member Is Offline
Mood: Have no fear <Vlad> is here.
|
|
Serious question:
Mixture of PETN with Nitromethane...
i have heard, that NM destroys the PETN over some time....any answeres?
|
|
|
hissingnoise
International Hazard
    
Posts: 3940
Registered: 26-12-2002
Member Is Offline
Mood: Pulverulescent!
|
|
Why would you want to compromise the performance and stability of PETN by mixing with a nitro-compound like NM?
|
|
|
VladimirLem
Hazard to Others
  
Posts: 204
Registered: 24-5-2010
Member Is Offline
Mood: Have no fear <Vlad> is here.
|
|
+ NM is more powerful than AN
+ dust-dry PETN is hard to densify
+ i cant get a higher desnity with wax or 10% water because:
- Im not the kind of guys, who are able to build Explosive Devices with -pure- PETN...
Most times, around 70 up to 90 percent are AN 
Mixtures of AN (50-75%) NM (20-30%) and some HE (TNP, PETN, MHN) make a cheap, strong explosive.
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
There it is again , Nitroguanidne forms a Nitrate salt adduct
Thanks to Rosco Bodine for this gem
The energetic double salt nitroguanidinium nitrate guanidinium nitrate
http://www.sciencemadness.org/talk/files.php?pid=217632&...
.
|
|
|
|