Antwain
Hazard to Others
  
Posts: 252
Registered: 21-7-2007
Location: Australia
Member Is Offline
Mood: Supersaturated
|
|
Isolating dimethylhydantoin
Some time ago I produced bromine by reduction of BCDMH with Na2SO3. I kept the residue for isolating dimethylhydantoin. I neutralised the acid
produced in the reaction by adding NaHCO3 until no more fizzing occurred, but nothing is precipitating. Very little had crystallised before but I
simply put this down to the formation of 'onium' salts in the acidic solution. Does anyone know why I am still getting no precipitate? DMH is rather
insoluble and there should be the best part of 100g in a liter of solution. Is it possible that it was hydrolised or in some way destroyed by leaving
it in acid solution for so long?
|
|
|
chemrox
International Hazard
    
Posts: 2961
Registered: 18-1-2007
Location: UTM
Member Is Offline
Mood: LaGrangian
|
|
| Quote: | | Originally posted by AntwainIs it possible that it was hydrolised or in some way destroyed by leaving it in acid solution for so long?
|
How long was it in acid solution? Also "dimethylhydantoin" is a little vague. Is it N,N' dimethylhydantoin, 1,5 0r 3,5 ?
You said you added base until fizzing stopped. Did you check the pH? Carbonyl compounds are complicated by the fact that under basic conditions they
are electrophillic at at the carbonyl C and nucleophillic at the a-C. You might have deprotonated the a-C with excess base and made the enolate. You
could try neutralizing the solution or making it slightly acidic and see what happens.
[Edited on 17-11-2007 by chemrox]
"When you let the dumbasses vote you end up with populism followed by autocracy and getting back is a bitch." Plato (sort of)
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
BCDMH was given as the origin, so it would be 5,5 dimethylhydantoin
|
|
|
chemrox
International Hazard
    
Posts: 2961
Registered: 18-1-2007
Location: UTM
Member Is Offline
Mood: LaGrangian
|
|
OIC-thanks N_I I missed that. That eliminates deprotanation at the a-C doesn't it? You could have the enolamine forming with two good resonance
structures. Ugh. I've had all sorts of troubles with heterocyclic carbonyls in basic solutions
[Edited on 17-11-2007 by chemrox]
"When you let the dumbasses vote you end up with populism followed by autocracy and getting back is a bitch." Plato (sort of)
|
|
|
The_Davster
A pnictogen
      
Posts: 2861
Registered: 18-11-2003
Member Is Offline
Mood: .
|
|
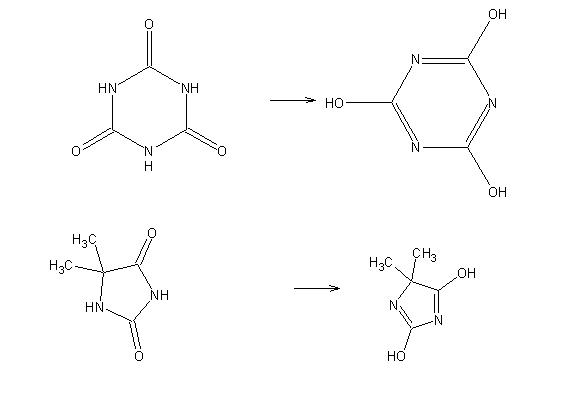
Well TCCA when it looses its chlorines ends up at cyanuric acid. Cyanuric acid can form salts.
Perhaps something similar happens when dimethylhydantoin is under basic conditions. Cyanuric acid forms salts, why not the lower right structure
below doing the same?
Perhaps carefully acidifying?
[Edited on 18-11-2007 by The_Davster]
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
According to http://www.cfsan.fda.gov/~acrobat2/fnea0308.pdf DMH has a solubility of 135 g/l in water at what I assume is 25 C. It's resistant to hydrolysis
in the pH range 5 to 9, no info for outside that range.
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
It won't hydrolyze at room temperature even at pH 1.
There was no need to neutralize, but it won't hurt either as its pKa1 is about 9 so even if you added excess NaHCO3 it will barely increase any
solubility by deprotonation. A more important thing would be to quench all the remaining oxidant species with sulfite or whatever.
Extraction is not wise due to the unfavorable partition coefficients. So you are left with either:
A.) evaporating the water out, extracting the solids with ethanol (or acetone, i-PrOH or what you have), evaporating the solvent and recrystallization
from water;
B.) saturating with NaCl, waiting for 5,5-dimethylhydantoin to crystallize out, filtering and recrystallization.
Of course, method A would give you more product, but evaporating a liter of water down without using vacuum sucks a lot.
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by Nicodem
...
A.) evaporating the water out, extracting the solids with ethanol (or acetone, i-PrOH or what you have), evaporating the solvent and recrystallization
from water;
... |
Do you have solubility information for solvents other than water?
|
|
|
leu
Hazard to Others
  
Posts: 368
Registered: 13-10-2005
Member Is Offline
Mood: No Mood
|
|
It's listed as very soluble in acetone, alcohol, benzene, chloroform and ether so it seems it's probably soluble in most every organic solvent except
possibly the most highly non polar hydrocarbons 
Chemistry is our Covalent Bond
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
Sounds like evaporation, perhaps helped along by an aspirator, followed by extraction with organic solvent is the way to go.
|
|
|
Antwain
Hazard to Others
  
Posts: 252
Registered: 21-7-2007
Location: Australia
Member Is Offline
Mood: Supersaturated
|
|
Thanks guys.
| Quote: | Originally posted by Nicodem
It won't hydrolyze at room temperature even at pH 1.
There was no need to neutralize, but it won't hurt either as its pKa1 is about 9 so even if you added excess NaHCO3 it will barely increase any
solubility by deprotonation. A more important thing would be to quench all the remaining oxidant species with sulfite or whatever.
Extraction is not wise due to the unfavorable partition coefficients. So you are left with either:
A.) evaporating the water out, extracting the solids with ethanol (or acetone, i-PrOH or what you have), evaporating the solvent and recrystallization
from water;
B.) saturating with NaCl, waiting for 5,5-dimethylhydantoin to crystallize out, filtering and recrystallization.
Of course, method A would give you more product, but evaporating a liter of water down without using vacuum sucks a lot. |
Yes I neutralised bromine with sulfite prior to adding bicarbonate. I also deliberately chose bicarbonate so that the pH wouldn't be too high, I knew
about the rather acidic proton alpha to 2 carbonals.
I couldn't find the solubility of DMH in water, but I thought that it was far lower. That alone probably explains the solubility. Evaporating water is
simple with a shallow dish and if it must be done quickly a fan blowing across the surface. Evaporating water under vacuum is tricker for me, as you
can't use a solvent trap for fear of expansion and cracking, and letting it go through a pump is out of the question. Using a rotorvap is great, but I
don't have one myself.
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
You can live without a rotavapor if you have a magnetic stirrer and an aspirator. You just connect your flask to the aspirator and heat the flask in a
bath on the stirrer while stirring intensively. Works great for amateurs! Of course you can also set up a trap in between the flask and aspirator for
cases where you expect the solvent bumping out.
|
|
|