Douchermann
Hazard to Others
  
Posts: 117
Registered: 11-10-2005
Location: Illinois, USA
Member Is Offline
Mood: No Mood
|
|
Stability of sodium azide in solution?
Hey guys, I was wondering if sodium azide would decompose if it was in boiling distilled water. I have an air bag module (I seperated the firing
chamber from the actual bag) and it's impossible to open. I was hoping to let it sit in boiling water for some time to hopefully dissolve all the
sodium azide out. And also, would boiling down to reduce the volume decompose it? What is the solubility at those temperatures, I can only find 17C.
I would be using distilled water by the way. Thanks guys.
It\'s better to be pissed off than to be pissed on.
|
|
|
PainKilla
Hazard to Others
  
Posts: 306
Registered: 29-4-2004
Member Is Offline
Mood: No Mood
|
|
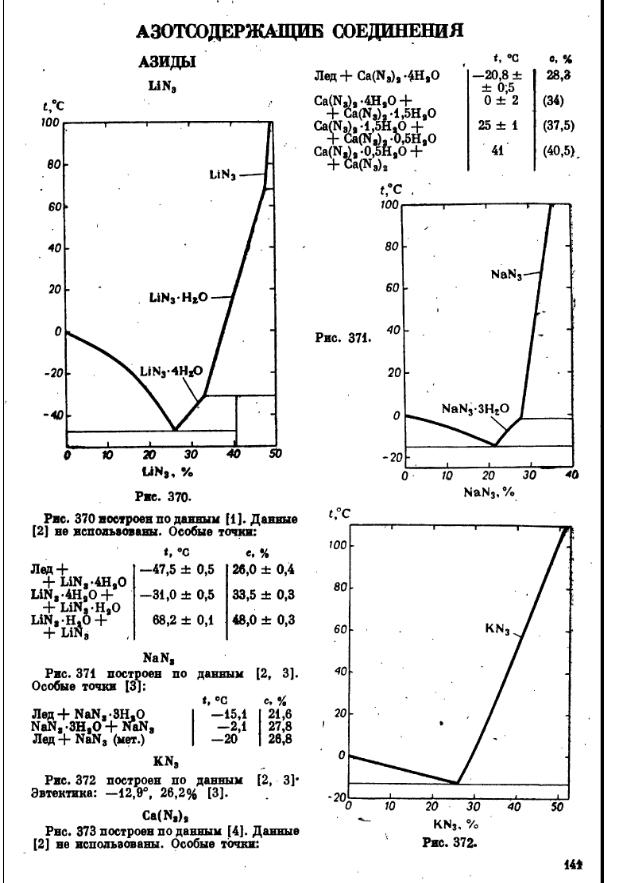
I don't have much to add other than the solubility data.
Edit:
These references should have the data on the stability of sodium azide.
Thermochimica Acta. 284 (2): 441-444.
Gakkaishi. 51(3):148.
[Edited on 11-12-2007 by PainKilla]
|
|
|
PainKilla
Hazard to Others
  
Posts: 306
Registered: 29-4-2004
Member Is Offline
Mood: No Mood
|
|
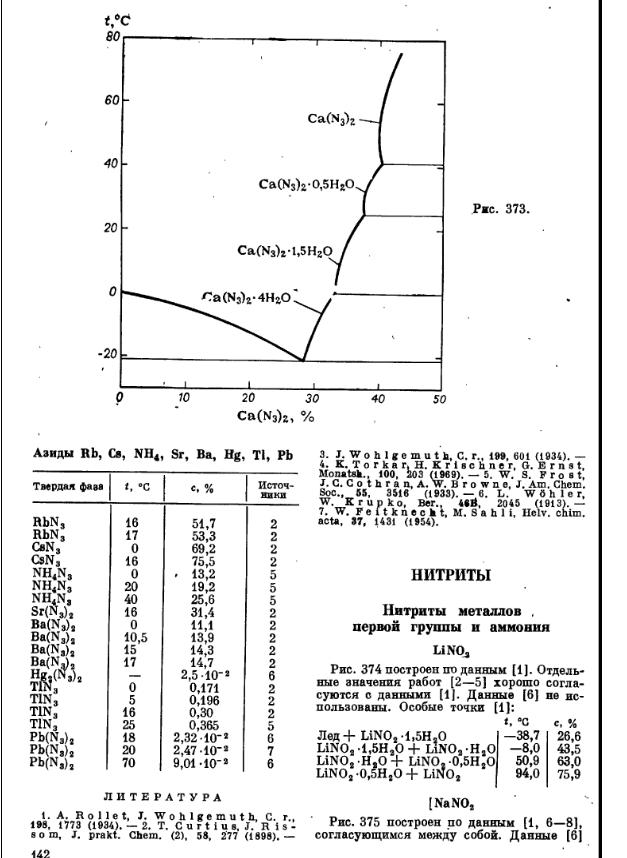
More info on azides, and the references:
|
|
|
PainKilla
Hazard to Others
  
Posts: 306
Registered: 29-4-2004
Member Is Offline
Mood: No Mood
|
|
Thermal Stability of Sodium Azide (Gakkaishi. 51(3):148.)
Yasuhiro FUJIMOTO, Takayuki ANDO and Shigeru MORISAKI
Abstract : Though chemical accidents have occured over and over, some part of these accidents are based on unstable chemical substances. These
chemicals easily decomose or igniteby heats or mechanical shocks under an atmosphere of not so much high temperature.
In this paper, sodium azide was chosen as an example of unstable chemical substances. Sodium azide is used in the preparation of hydrazoic acid,
lead azide, pure sodium or as propellant for inflating automotive safety bags, etc. It is no doubt that sodium azide is more stable than other azides,
however, the danger not been assessed satisfactorily.
Thermal decomposition of sodium azide is shown as follows.
2NaN3 ==> 2Na + 3N2 (1)
It is known that the decomposition takes place at 300*C. In addition, the heat of decomposition has been reported. However, these results were
obtained about half a century ago and the other thermal analytical results, in particular under an adiabatic condition, have seldom been reported ever
since.
And sodium azide reacts chemically active organic halides as follows.
RCl + NaN3 ==> RN3 + NaCl (2)
But reactions between sodium azide and organic halide polymers have not been reported yet. Hence, this paper describes the results of the
following two subjects about thermal stability of sodium azide.
1. Thermal decomposition of sodium azide
(1) Decomposition temperature and heat of decomposition
The experiments were carried out by DSC in air and argon atmosphere. The decomposition temperatures were about 430 *C in air and about
400*C in argon atmosphere. The heats of decomposition were about 90 kcal/mol in air and about 10 kcal/mol in argon. Obviously, the mechanism
of thermal decomposition in air is different from that in argon. This is because metal sodium, which is produced through thermal decompositon of
sodium azide, is oxidized by oxygen in air.
(2) Weight decrease by thermal decomposition
The experiments were conducted with TG-DTA in the above two different atmospheres. In air, the weight goes back considerably after a decrease in
the weight by the decomposition of sodium azide to pure sodium and nitrogen. But in argon, such a phenomenon was not observed.
These results show that the sodium is certainly oxidized in air after decomposing of sodium azide. The following reaction scheme is estimated
according to the above results.
4NaN3 + 02 ==> 2Na2O + 6N2 (3)
(3) Self-heat rate and pressure under an adiabatic condition
The experiment under an adiabatic condition was carried by ARC only in an inert gas. Beginning of self-heat of sodium azide was observed at around
335*C, and the following decomposition was too fast to follow the increase in the self-heat rate with ARC.
2. Reactions between sodium azide and organic halide polymers
(1) Measurements under programmed temperature
DSC was also used for the experiments in an atmosphere of air. The decomposition temperatures of any organic polymers, such as polyethylene,
polypropylene, polyvinylchloride and polytetrafluoroethylene, were not changed in the presence of sodium azide, and no DSC-peaks due to exothermic
decomposition were observed.
(2) Measurements under fixed temperatures
The experiments were carried out by DSC for providing another evidence in confirmation of no reactions between organic halide polymers and sodium
azide. No exotherms were observed at the constant temperature of 300*C for 2 hrs which might arize by the reactions between the polymers and the
azide. The above results show surely that sodium azide may be thermally stable with organic polymers at relatively high temperature.
[Edited on 11-12-2007 by PainKilla]
|
|
|
chemrox
International Hazard
    
Posts: 2961
Registered: 18-1-2007
Location: UTM
Member Is Offline
Mood: LaGrangian
|
|
I wonder how relevant these data are considering he proposes to place the material in boiling water? It doesn't seem likely that thermal
decomposition at 300 deg. is going to happen at 100 deg in water. It is an ionic substance so trinitride ion is the species involved as soon as water
gets to work on it. I suppose since it's an airbag the material could get heated a bit before solvation but not to anywhere near 300. Isn't there
some safety data on it? What is supposed to happen to it if a car catches fire? Must be a lot less of an issue than the fuel.
"When you let the dumbasses vote you end up with populism followed by autocracy and getting back is a bitch." Plato (sort of)
|
|
|
Nick F
Hazard to Others
  
Posts: 439
Registered: 7-9-2002
Member Is Offline
Mood: No Mood
|
|
When I got azide from airbags I just used a saw to get it out... 30 mins of careful sawing and it'll be done (assuming they're all more or less
standard in their construction). That's less time than it'll take you to boil down the water and crystalise the sodium azide using your poposed method
(although I don't doubt that what you suggest will work).
|
|
|
Nick F
Hazard to Others
  
Posts: 439
Registered: 7-9-2002
Member Is Offline
Mood: No Mood
|
|
P.S. - http://en.wikipedia.org/wiki/Solubility_table
It's quite useful, although in this case it doesn't give much info.
|
|
|
chemoleo
Biochemicus Energeticus
    
Posts: 3005
Registered: 23-7-2003
Location: England Germany
Member Is Offline
Mood: crystalline
|
|
I wouldnt recommend it.
NaN3 is used in biochem for preventing fungal/bacterial growth. Usually a 0.1% solution is used. It is always said though that these solutions don't
last long, a few weeks at the very most, at 4 deg. I wouldnt want to think about what happens when you boil it.
Try to precipitate it with i.e. Pb and resolubilise with (without ever working with the dry material) NaOH? The PbOH2 thus formed is insoluble.
Never Stop to Begin, and Never Begin to Stop...
Tolerance is good. But not with the intolerant! (Wilhelm Busch)
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
and there is this
http://www.geneseo.edu/~ehs/Lab%20and%20Art%20Studio%20Safet...
http://www.ehs.neu.edu/hazardous_waste/fact_sheets/sodium_az...
|
|
|
Tinton
Harmless

Posts: 15
Registered: 16-12-2007
Member Is Offline
Mood: homework laden
|
|
NaN3 forms small amounts of hydrogen azide with water. Actually i've heard NaN3 + H2O is used to produce HN3, but I've also heard that only trace
amounts of HN3 are produced.
Apparently a KNO3/NaN3 mixure is used, not just NaN3
I'm sorry I couldn't give you something more useful, like a solubility table.
But, just out of amateur curiosity, why not use a ketone or alcohol as the solvent?
|
|
|