woelen
Super Administrator
        
Posts: 7976
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
If you like colored gases... Interhalogens such as Bromine chloride, BrCl, ClBr
This is a set of experiments which is quite interesting to see. The halogens also react with each other quickly and the resulting compounds are quite
interesting to see. Br2 and Cl2 react with each other in a 1 : 1 molar ratio, giving a beautiful golden yellow gas:

I never had seen such an amazing color for a gas, but it is really easy to make, provided you have access to bromine.
http://woelen.homescience.net/science/chem/exps/raw_material...
If you do these experiments, do them in a fume hood or a very well ventilated place with a lot of draught, such that the gases cannot be inhaled.
Edited title. Chemoleo
[Edited on 1-3-2008 by chemoleo]
EDIT by woelen: Changed URL's of links, so that they work again.
[Edited on 12-6-12 by woelen]
|
|
|
guy
National Hazard
   
Posts: 982
Registered: 14-4-2004
Location: California, USA
Member Is Offline
Mood: Catalytic!
|
|
Are you sure its not just a mixture of the colors?
|
|
|
woelen
Super Administrator
        
Posts: 7976
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
A mix of such colors looks different. Just keep a layer of bromine and a layer of chlorine behind each other, and you don't get the same color (it
will be darker, much more reddish). Also, look at the difference when you put some bromine in a bottle, filled with air, and you put the same amount
of bromine in a bottle with chlorine. In the latter case, the color of the gas mix becomes lighter and more yellow, and the bromine 'evaporates' much
faster. Bromine chloride is a known compound.
http://en.wikipedia.org/wiki/Bromine_monochloride
I also have a book, "chemistry of the elements" and this also mentions this as being a volatile compound with a yellow/brown color, which fairly
easily dissociates. But in a closed vessel with a little excess of chlorine, it definitely exists and has rather different color than a mix of Br2 and
Cl2.
|
|
|
chemoleo
Biochemicus Energeticus
    
Posts: 3005
Registered: 23-7-2003
Location: England Germany
Member Is Offline
Mood: crystalline
|
|
Wow, very nice. Are there some specific reactions that can be done with BrCl but not Cl2, or Br2?
I wonder what the cause is for this clearly exothermic favorable reaction.
One gives up energy, the other receives it, which should be the same vice versa. That is, the Cl-Cl bond is of higher energy than that of Br-Br, so
swapping partners should cancel energies out right? But then, things are always in equilibrium...so I'm sure there'll be some leftover Cl2 and Br2.
Perhaps it would be interesting to investigate this with a spectrophotometer, since both compounds absorb in the visible range, and since it seems
feasible making such an apparatus with LEDs (see other threads)?
Oh, and I renamed your thread slightly for future searchers of this forum.
[Edited on 23-12-2007 by chemoleo]
Never Stop to Begin, and Never Begin to Stop...
Tolerance is good. But not with the intolerant! (Wilhelm Busch)
|
|
|
JohnWW
International Hazard
    
Posts: 2849
Registered: 27-7-2004
Location: New Zealand
Member Is Offline
Mood: No Mood
|
|
Another possible product, more likely with an excess of Cl2, is BrCl3.
|
|
|
YT2095
International Hazard
    
Posts: 1091
Registered: 31-5-2003
Location: Just left of Europe and down a bit.
Member Is Offline
Mood: within Nominal Parameters
|
|
| Quote: | Originally posted by woelen
and the bromine 'evaporates' much faster. |
have you tried it with I2?
I imagine it would take Longer that the Br2 to go into gas, but if the Cl2 is acting as a type of "Solvent" I wonder what color you will get with
Iodine? 
\"In a world full of wonders mankind has managed to invent boredom\" - Death
Twinkies don\'t have a shelf life. They have a half-life! -Caine (a friend of mine)
|
|
|
woelen
Super Administrator
        
Posts: 7976
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
@YT205: I tried with I2, that is in the webpage, to which I provided a link. The Cl2 indeed liquefies the I2 to a brown liquid (ICl) and if a lot of
Cl2 is added, it evaporates, giving rise to a yellow solid (ICl3).
@JohnWW: The compound BrCl3 is non-existent at macroscopic level, at least according to my books. Of iodine, bromine and chlorine, the only
interhalogens, which are mentioned as being sufficiently stable to have them in more than trace amounts under normal conditions are BrCl, ICl, ICl3.
IBr is somewhat intermediate, it does exist in a bromine/iodine mix, but only in small amounts in a labile equilibrium.
@chemoleo: BrCl does show a somewhat different reaction than Cl2 or Br2 alone. With water, it results in formation of noticeable amounts of HCl
(fuming) and formation of bromic acid. At higher concentrations, however, the reaction is reversed again. This also is an equilibrium. This effect is
even stronger with ICl. With water, that compound really fumes, giving HCl and iodic acid.
|
|
|
JohnWW
International Hazard
    
Posts: 2849
Registered: 27-7-2004
Location: New Zealand
Member Is Offline
Mood: No Mood
|
|
Of course, if you added F2 (dangerous - do not try it at home!) to the mixture, the number of possible interhalogen compounds increases greatly. In
the case of compounds with iodine, there is IF (detected only in vapor), IF3, IF5, IF7, of which the last is the favored product. In the case of
compounds with chlorine, there is ClF, ClF3, ClF5; and not long ago the cation ClF6+ of Cl(VII) (as the hexafluoroantimonate) was obtained by reaction
of ClF5 with [KrF][SbF6].
|
|
|
woelen
Super Administrator
        
Posts: 7976
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
I had supplied wrong information in my webpage and in previous posts. The compound IBr does exist and is fairly stable. I was mistaken with
information from an older book, but according to my new 1997 book, "Chemistry of the elements", IBr is a fairly stable compound, which melts at 41 C
and boils at 116 C (with decomposition). It is deep red, almost black. Its vapor is also deep red, when diluted it becomes beautifully brick red. Even
at room temperature, there still is appreciable vapor from this compound, and its color differs quite a lot from the color of bromine vapor.
I modified my webpage, adding the experiment of IBr and I like this result also quite a lot:
http://woelen.homescience.net/science/chem/exps/raw_material...
A picture from that experiment is shown here (test tubes here are warm, not really hot, and contain rather dilute vapors of bromine, iodine bromide
and iodine from left to right):

EDIT by woelen: Changed links, so that they work again.
[Edited on 12-6-12 by woelen]
|
|
|
Jdurg
Hazard to Others
  
Posts: 220
Registered: 10-6-2006
Location: Connecticut, USA
Member Is Offline
Mood: No Mood
|
|
Wow Woelen. The deep, intense colors of the bromine, Iodine bromide, and Iodine vapors directly after the intense heating are amazing. I've never
seen such deep, intense colors. Simply beautiful. It reminds me of the first time I saw liquid Iodine and how stunned I was at how amazingly purple
it was.
\"A real fart is beefy, has a density greater than or equal to the air surrounding it, consists of the unmistakable scent of broccoli, and usually
requires wiping afterwards.\"
http://maddox.xmission.com. |
|
|
chemoleo
Biochemicus Energeticus
    
Posts: 3005
Registered: 23-7-2003
Location: England Germany
Member Is Offline
Mood: crystalline
|
|
NOt to be a party pooper ... but what is the colour of transmitted light if you hold the left and right testtube right behind each other, compared to
the one with truly mixed Br2/I2?
Never Stop to Begin, and Never Begin to Stop...
Tolerance is good. But not with the intolerant! (Wilhelm Busch)
|
|
|
len1
National Hazard
   
Posts: 595
Registered: 1-3-2007
Member Is Offline
Mood: NZ 1 (goal) - Italy 1 (dive)
|
|
Very pretty - but, I need more convincing. What chemoleo says isnt enough because a mixture of I2/Br2 in the gas phase need not be equimolar. It
would depend on the partial pressures, so holding one testube behind the other might not be enough.
What you need to do is show us the mixture before reaction - and then after. Alternatively since the compound decomposes at 116, there should be a
colour change at that temperature - that will definitely demonstrate the existance of a compound.
|
|
|
woelen
Super Administrator
        
Posts: 7976
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
What i think is quite convincing is that the test tube with IBr in it even at room temperature has a clearly visible vapor in it, which has a color
quite different from bromine vapor, while the testtube with iodine has hardly any visible vapor in it.

If you would take the test tube at the left and the one at the right, then it would hardly differ from only the test tube at the left. The middle one
has a color intensity, somewhat similar to the left one, but its color is much more reddish. If no reaction would occur between iodine and bromine,
then the middle one also would get a color like the left one on cooling down (the iodine would become solid again, just as in the right test tube,
while the bromine would remain in vapor phase).
Another fairly convincing observation is that IBr is present on the Fisher/Emergolab catalogue at a purity of 98%  . But I only discovered that last fact two days ago. . But I only discovered that last fact two days ago.
[Edited on 12-6-12 by woelen]
|
|
|
len1
National Hazard
   
Posts: 595
Registered: 1-3-2007
Member Is Offline
Mood: NZ 1 (goal) - Italy 1 (dive)
|
|
Thanks woelen. I dont want to sound mean, but Im still not convinced. This could be explained by co-volatilization - I2 is more volatile in the
presence of bromine - the same type of thing that leads to the formation of azeotropes - which are mixtures - not compounds.
Did you get any heat change when you mixed Br2 and I2? That can be a sign of chemical reaction.
Sure such a compound exists - but thats not enough to prove that you have it. I still think observing a colour change at the decompisition
temperature would be definite proof. Len
PS Theres another way to prove the existance of a Br-I bond. If you do a spectrum of the mixture/compound and find the colour we see is due to
absorption bands different from those of either I2 and Br2, rather than a superposition of them, theres your proof.
[Edited on 13-1-2008 by len1]
|
|
|
woelen
Super Administrator
        
Posts: 7976
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
Len1, you are right, I cannot really proof things, I can only give evidence for the presence of such a compound. I wish I had the equipment to real
spectrum measurements. The average home chemist, however, does not have that kind of equipment. Literature tells that Br2 and I2 are in equilibrium
with IBr (see ref in my webpage), with by far the largest part being IBr at room temperature. My demo also was not intended as being a proof of
something, but more to show that nice gaseous compounds can be made with interesting colors.
I could do the test of heating above 116 C, but at those temperatures, the color of the vapor is really dense and any changes are hard to observe. I
think that changes are hard to observe anyway, a real proof indeed is the spectrum measurement. In that way, the compound BrCl was discovered, more
than 100 years ago. They found that a mix of Br2 and Cl2 (which has a nice yellow color) absorbs in the UV-range, while neither Br2 nor Cl2 does. This
is a clear indication that there is a new compound. Of course it does not tell which percentage of Br2 and Cl2 have reacted, but it surely tells that
there is a reaction.
|
|
|
microcosmicus
Hazard to Others
  
Posts: 287
Registered: 31-12-2007
Member Is Offline
Mood: spin up
|
|
Does your reference say where in the UV-range BrCl absorbs?
Given a suitable UV lamp and fluorescent paper, one could try
holding a tube of the gas between the lamp and the screen and
seeing whether it casts a noticeable shadow. Since ordinary
glass also absorbs in UV range, it might be necessarry to use
a container made out of some other substance or pick one
with walls thin enough not to absorb too much UV.
|
|
|
len1
National Hazard
   
Posts: 595
Registered: 1-3-2007
Member Is Offline
Mood: NZ 1 (goal) - Italy 1 (dive)
|
|
Ill put that in the to do basket. I have a UV/visible/NIR spectrometer, but the challange with amateur chemistry is to prove something with the
minimum of equipment - its amazing what can be done. The amateur chemist here does not have access to iodine - and im not sure if one is allowed to
make it from the tincture - ill check, so I might just have to investigate the bromine.
PS Ive seen your website - very nice. Are you a chemistry teacher? Len
|
|
|
chemoleo
Biochemicus Energeticus
    
Posts: 3005
Registered: 23-7-2003
Location: England Germany
Member Is Offline
Mood: crystalline
|
|
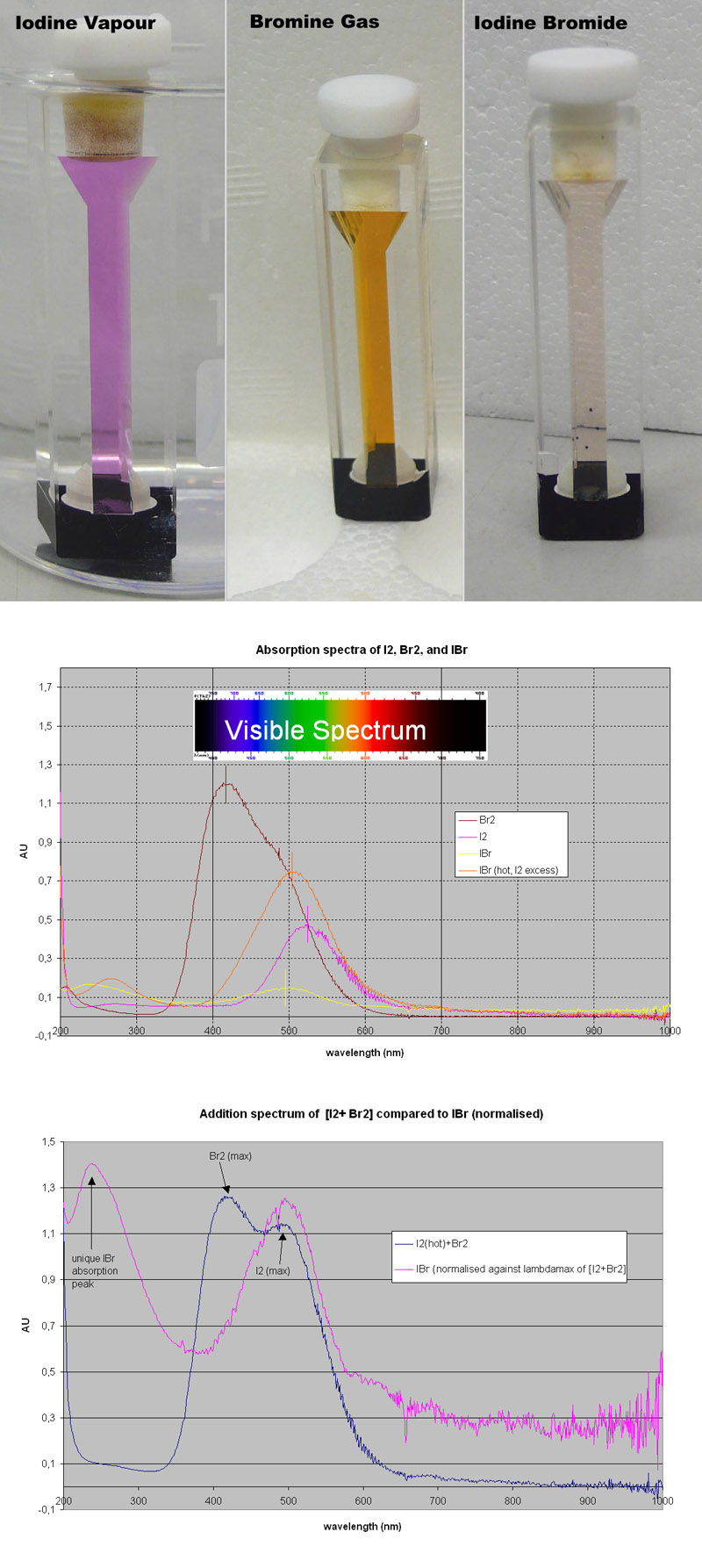
Interhalogen absorption spectra
I got round to measuring the absorption spectra of I2, Br2 and putative IBr.
The objective was to see whether the absorption spectrum of the mixed product of I2 + Br2 --> 2 IBr is simply a mixture (which should
provide an addition spectrum of each component I2 and Br2) or a unique spectrum which does not resemble the addition product of the spectra of the
individual elements!
I2 was analytical grade (crystals), and dense Br2 gas was generated by adding solid calcium hypochlorite to a solution of potassium bromide and
hydrochloric acid. The KBr dissolved very badly in 20% HCl ([almost] common ion effect?), but KBr dissolved very well in H2O without acid. This
allowed for better concentrations of Bromide, and the resulting Br2 vapours emanating from the reaction vessel were very dense.
In fact so dense it could be sucked up (aspirated to use a more scientific term  ) almost like a liquid using a pipette. ) almost like a liquid using a pipette.
In the first picture left you can see Iodine vapour in the cuvette (quartz, pathlength 1 cm, the whole cuvette is about 4 cm tall).
Here a few crystals of I2 were simply added to the cuvette. Iodine vapour at RT was virtually undetectable in the spectrophotometer, so it was heated
in a waterbath at 90 deg C, and the spectrum was then taken immediately (had to be quick, condensation was very fast!). The pic was taken in the hot
waterbath beaker, hence the slight distortion.
In the middle picture this is bromine vapour, pipetted directly from the reaction vessel into the cuvette. This one absorbed nicely.
The right pic is the cuvette containing Iodine bromide, obtained by shaking the iodine crystals/vapour directely into the bromine
vapour, hardly any gas was able to escape due to the density of the I2/Br2 vapours (meaning that paleness of the I2/Br2 picture is not an effect of
dilution with air). Interestingly, the colour immediately changed to a paler version, as shown in the pic. This was very striking, and to me evidence
that a new compound was generated, and not just a mixture of the gasses.
Of each of the gasses, I2 (hot), Br2, IBr (RT), IBr (hot, with I2 in excess as I2 crystals were in cuvette) an absorption spectrum was taken betw
200-1000 nm, which covers the visible light spectrum and beyond. These were blanked against air.
The overlaid spectra are shown in picture 2.
AU means Absorption Units, a unit derived from the Beer Lambert law.
I was very pleased with this as a difference is very clear, Br2 absorbs at RT like crazy, I2 (hot) too, but quite differently, while the mix of the
two gasses (IBr) absorbs very little (also quite clear from the pic top right).
More importantly, IBr has a different absorption maximum (lambda max), around 490 nm, while I2 has a maximum at 530 nm. This is clearly
indicative of a new compound.
This is evaluated more in picture 3, where the absorption values of Br2 and I2 (the latter normalised against lambdamax of Br2) are
added up (blue), and plotted against the wavelength. Compared to IBr (hot, meaning there is a good fraction of I2) this spectrum (purple) looks very
different compared to the addition spectrum of I2 and Br2.
1) The Br2 lambdamax (420 nm) is missing in the IBr spectrum
2) A new peak appears at ~230 nm, which is not seen in either I2 or Br2 spectra.
Also, note the overlay of the visible spectrum and their colours in picture 2:
Br2 absorbs from blue to green, leaving largely red. THis is what makes bromine brown.
I2 on the other hand absorbs largely in the green range, leaving largely red and a bit of blue. Hence the beautiful purple colour of iodine!
Whilst IBr absorbs more towards the blue/green part of the spectrum than I2, making the interhalogen less purple and more brownish!
Together, I think we can safely conclude that a new compound is generated by combining I2 and Br2 vapours, which presumably is IBr!
Woelen, thanks for getting me interested in this, this was a very nice experiment to do. If there are other reproducible experiments (not necessarily
interhalogens) that need clarifying with a spectrophotometer, then let me know, I'll be glad to assist!
[Edited on 10-3-2008 by chemoleo]
Never Stop to Begin, and Never Begin to Stop...
Tolerance is good. But not with the intolerant! (Wilhelm Busch)
|
|
|
MagicJigPipe
International Hazard
    
Posts: 1554
Registered: 19-9-2007
Location: USA
Member Is Offline
Mood: Suspicious
|
|
In support of your results I found this article that I can't access but here's the google summary (the first hit when I searched for "iodobromide
interhalogen".
Electron absorption spectra of the halogens in the tetrahalides of ... Iodobromide was synthesized by direct combination of the two halogens, the ....
Interhalogen Compounds [/n Russian], Izd-vo AN Ukr. 8Sl Kiev (1958).
Maybe someone has access to the article and can provide more information.
"There must be no barriers to freedom of inquiry ... There is no place for dogma in science. The scientist is free, and must be free to ask any
question, to doubt any assertion, to seek for any evidence, to correct any errors. ... We know that the only way to avoid error is to detect it and
that the only way to detect it is to be free to inquire. And we know that as long as men are free to ask what they must, free to say what they think,
free to think what they will, freedom can never be lost, and science can never regress." -J. Robert Oppenheimer
|
|
|
The_Davster
A pnictogen
      
Posts: 2861
Registered: 18-11-2003
Member Is Offline
Mood: .
|
|
Can't believe I missed the whole argument on whether or not it exists!
In any case, I synthesized IBr in my inorganic lab a few years ago. IIRC it was direct combination of I2 and a slight excess of Br2, and then the
product was analysed by UV/VIS. You know, exactly the work replicated here  . .
It reminded me of iodine in that it was a shiny solid but much more volatile and the vapours more red.
[Edited on 9-3-2008 by The_Davster]
|
|
|
woelen
Super Administrator
        
Posts: 7976
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
Chemoleo, that is very interesting. Many thanks for the work you have done!
I already was inclined to think that IBr is a compound, but this is a very good confirmation. On the eye, one can see some differences, but this
indeed could be a mix of the colors, although the fact that the vapor is more dense and volatile is an indication (not a proof!) that a new compound
is formed.
I've never heard of another iodine bromide besides IBr. I have the book 'Chemistry of the elements' and it mentions the following possible
non-fluoride interhalogen compounds:
- ICl
- ICl3
- IBr
- BrCl
It could be that IBr changes color, when its temperature changes. I know quite a few solids which have this property (e.g. PbI2, yellow when cold.
brick-red when hot, K2CrO4, almost white when very cold, yellow when at room temperature, orange when hot). The special thing about IBr then would be
that the temperature dependence of the color is in the gas phase.
|
|
|
Nick F
Hazard to Others
  
Posts: 439
Registered: 7-9-2002
Member Is Offline
Mood: No Mood
|
|
Nice work Chemoleo  . .
|
|
|