kclo4
National Hazard
   
Posts: 916
Registered: 11-12-2004
Location:
Member Is Offline
Mood: No Mood
|
|
Where, or how can i get Alagebrium?
http://en.wikipedia.org/wiki/Alt-711
I've tried to find a place to buy this chemical, and i cannot.
So, i suppose a synthesis is in need? It looks like it is going to be a bitch and a half to make to me at least, after all i am not experienced in
organic chemistry. Anyways, to me it seems like Acetophenone (Acetylbenzene) will be needed, as well as 4,5 Dimethyl Thiazole. Once you have those
two, i suppose all you need to do is bind the nitrogen to the methyl group of the Acetophenone. How to do that? i really have no clue.
This could possibly be useful for a synthesis towards making 4,5 thiazole.
http://en.wikipedia.org/wiki/Thiazole
Maybe the thiazole ring could be stolen from thiamine?
Thanks in advance 
[Edited on 28-12-2007 by kclo4]
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
This would not be a difficult preparation at all.
Undoubtedly the prep has been described in the literature. There is little need to be creative about that. Just look it up.
But a warning. If you are not an experienced organic chemist, you may not be up to the task, not of just making this, but of purifying it rigorously,
usually by HPLC. Without that you sound like you are proposing to ingest, or have someone else ingest, an experimental medication that will contain
who knows what impurities? Not a good idea. You could easily do more harm than good. Without the ability to purify and analyze you are blind and deaf.
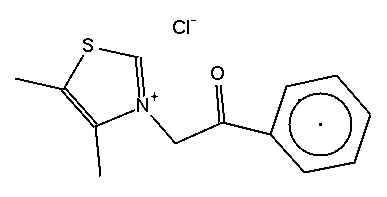
IUPAC for this is 2-(4,5-Dimethyl-thiazol-3-yl)-1-phe
nyl-ethanone. Does not fall trippingly off the tongue.
3-phenacyl-4,5-dimethylthiazolium chloride is much less correct but at least more manageable to speak, and still clear.
To make the thiazolium, start with MEK. Brominate this at the alpha position on the ethyl side. The stuff will be lachrymatory.
React the carbonyl with hydroxylamine hydrochloride. You now have an oxime.
Now react the Br with potassium ethyl xanthate in acetone.
Close the ring with ZnCl2 (anhydr.) in Et2O.
You now have 4,5-dimethylthiazole with a -OH group on the N and a thione at the 2-carbon to get rid of.
This is off the top of my head. The lit. may contain more elegant methods. When you see a heterocycle like this start thinking about thiourea or
isothiouronium salts, thioacetamine, that sort of thing. Mostly this is 19th century stuff, so bone up on your German.
Once you have 4,5-simethylthiazole, getting it to react at the ring N with the side chain C3 of acetophenone is trivial.
This compound is being investigated as a reversal agent for the effects on diabetes, so as a diabetic I am quite interested. Retinopathy, nephropathy,
neuropathy are words I know well.
[Edited on 25-12-2007 by Sauron]
Sic gorgeamus a los subjectatus nunc.
|
|
|
Nick F
Hazard to Others
  
Posts: 439
Registered: 7-9-2002
Member Is Offline
Mood: No Mood
|
|
From the little bit that I read it sounds like it disrupts protein crosslinks caused by carbohydrate linkages.
I never studied protein chemistry to any significant level, but are there not intended carbohydrate crosslinks which this could fuck up? I don't know,
it's Christmas and I'm a bit drunk :p.
The synthesis would be pretty straight forward, possibly even kitchen do-able (in a well-equiped kitchen!), but yeah, purification would be a
stumbling block. This is probably especially true with bioactive things. If the desired product is bioactive, then it is quite possible that similarly
structured by-products will be also, though not neccesarily in a good way... eg what if S linked to the aromatic side chain? Might be harmless, might
be teratogenic... What I mean is, purifying something to 99.9% is easily do-able at home in many cases, but if it's a bioactive and you intend to
ingest it then I would want much greater purity, just in case!
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
The precursors are nasty.
The patents are very broad and not especially helpful, but, one of them contains a lot of information of the preparation of the oxazoles analogous to
thiazoles.
I know that oxazoles can be thiated to thiazoles by the use of a reagent I have described on this forum previously, The reagent is prepared from
anisole and P4S10 as described in Org.Syn. It is preferable to using P4S10 itself in thiation.
The US patent 6596744B2 is the one to read.
Sic gorgeamus a los subjectatus nunc.
|
|
|
Ozone
International Hazard
    
Posts: 1269
Registered: 28-7-2005
Location: Good Olde USA
Member Is Offline
Mood: Integrated
|
|
Ths is, however, a round-about route to an excellent topic, that is Advanced Glycation Endproducts (AGEs).
AGEs are products of the reaction (see Maillard) between reducing sugar and an amino group (usually an amino acid appendage of protein, in-vivo). The
most reactive residues are arginine (with a guanidine end-group) and lysine (with the epsilon NH2 "dangling" out there).
The compound likely (see tenilsetam) prevents crosslinking via "pentosidine", "argpyrimidine" (dimers) or end capping leading to
carboxymethyl-Ne-Lysine. It also might serve as a cross-link breaker. See also metformin (glucophage)--which can give lethal side effects.
As Sauron wisely accounts, the prescribed dosage for these drugs (see metformin, aka. super-aminoguanidine) is very high (near stoichiometric, at
500mg and up), so even small traces of impurity could be very dangerous.
I have attached a .zip with a few (six) papers for your perusal. I'm off to visit the family.
OK, the zip is too big, let's try the first three.
Merry Christmas to all,
O3
[Edited on 25-12-2007 by Ozone]
Attachment: 1-3.zip (2.2MB)
This file has been downloaded 468 times
-Anyone who never made a mistake never tried anything new.
--Albert Einstein
|
|
|
Ozone
International Hazard
    
Posts: 1269
Registered: 28-7-2005
Location: Good Olde USA
Member Is Offline
Mood: Integrated
|
|
OK, that seemed to work. I have, literally, hundreds of full papers on this subject, so if you need one, let me know. If I have it, I will forward it
to you or post it here.
Part 2,
O3
Attachment: 4-6.zip (814kB)
This file has been downloaded 568 times
-Anyone who never made a mistake never tried anything new.
--Albert Einstein
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
Thanks, O3
The most facile prep of 4,5-dimethylthiazole I have been able to figure out is to start with MEK (methyl ethyl ketone), alpha chlorinate it with NCS
(N-chlorosuccinimide) to methyl 1-chloroethyl ketone. You will need to purify that to remove any chloromethyl ethyl ketone.
React the chloroketone with formamide. This will get you 4,5-dimethyloxazole. This is also commercially available.
Now you are ready to thiate the oxazole using Lawsson's reagent. Look up that in Org.Syn. You can buy it or make it from anisole and phosphorus
pentasulfide.
Lawsson's reagent replaces the O in the ring with S. Voila, you now have 4,5-dimethylthiazole.
To obtain the phenacyl substituted target compound, you need phenacyl chloride.
Huh? Phenacyl chloride = chloroacetophenone = CN tear gas. Well, it's not a gas, it's a solid (powder).
Yes, you make alagebrium out of CN tear gas.
CN is made by the Friedel Crafts acylation of benzene with chloroacetyl chloride catalyzed by AlCl3.
Chloroacetyl chloride is very nasty stuff, don't even think about handling it outside of a hood.
Once you have reacted your CN with the 4,5-dimethylthiazole, you still need to quaternize the alagebrium to its chloride quaternary salt.
And it's time for really rigorous purification.
I have to make some chloroacetyl chloride for another project. This involves treating chloroacetic acid with benzoyl chloride and distilling off the
chloroacetyl chloride. Definitely hood work.
All in all, while I would not call this preparation difficult in theory, in practice it is a serious pain in the ass. It involves not 1 but 3 serious
lachrymatory agents, one of which is included in the Chemical Warfare COnvention and thus can land you in trouble. That's the CN.
I would therefore advise waiting till this is on the pharm market.
Sic gorgeamus a los subjectatus nunc.
|
|
|
kclo4
National Hazard
   
Posts: 916
Registered: 11-12-2004
Location:
Member Is Offline
Mood: No Mood
|
|
Making this chemical seems to troublesome with all the dangerous and controlled substances involved.
So, i suppose the best thing to do would be to find something els easier to make that does nearly the same thing?
Well, O3 mentioned a few, how hard are they to make?
It seems there are a lot of AGE chemicals, Aspirin is even supposed to be one, according to the wikipedia on AGE
http://en.wikipedia.org/wiki/Advanced_glycation_endproduct
The reason i want this type of chemical is because i am planning on doing the <B>Methuselah Mouse Prize</B> www.mprize.org
All you basically try to do is make a mouse live significantly longer then the last winner.
When i do this, i want to try to fight most of the seven causes of aging. They are:
1. Cell loss or atrophy
2. Nuclear mutations
3. Mutant mitochondria
4. Cellular senescence
5. Extracellular cross-links
6. Junk outside cells
7. Junk inside cells
http://en.wikipedia.org/wiki/SENS
So, i suppose maybe just using aminoguanidine would be safer, cheaper, and easier to make simply from Nitroguanidine, HCl and zinc.
But I'd really prefer a more active compound that not only inhibits but can also break some cross link bonds like Alagebrium is claimed to do.
My parents want me to get off to open some presents, sorry if my post seems a tad bit crappy 
[Edited on 28-12-2007 by kclo4]
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
4,5-Dimethylthiazole, though expensive, is commercially available and unregulated, thus obtainable by individuals. Phenacyl chloride is easily
prepared by acetophenone chlorination with Cl2 in acetic acid (or with trichloroisocyanuric acid if you do not like "handling" Cl2). Or you can make
the chlorohydrine from styrene (being more OTC than acetophenone) and oxidize it to phenacyl chloride. Both the chlorohydrine formation and oxidation
are simple well documented in the literature. Dealing with phenacyl chloride is relatively easy compared to the much more volatile chloroacetone with
which lots of members here have experience in home-lab settings. I prepared and even distilled both in my amateur chemistry years and both are
annoying but can be worked with if all the reasonable precautions are used.
The alkylation of 4,5-dimethylthiazole with phenacyl chloride is relatively straightforward. The S-alkylated product is not a problem since
it readily equilibrates with Alagebrium(TM) which is the thermodynamic product. The good thing about using phenacyl chloride in the last step is in
that it is unforgiving in product purity demands. You will have to recrystallize really well to make it stop being irritant to the eyes.
Edit: If synthesizing 4,5-dimethylthiazole is more desired than buying it... It can be prepared by diazotizing 2-amino-4,5-dimethylthiazole and either
reducting the diazo salt with ethanol (which gives poor yields but is easy) or forming the 2-bromo-4,5-dimethylthiazole trough the Sandmeyer reaction
and reducing it with Zn/AcOH. 2-amino-4,5-dimethylthiazole itself is easily prepared from 3-chloro- or 3-bromo-butan-2-one and thiourea. For
references see J. V. Metzger: Thiazole and its derivatives (1979) (available as Djvu ebook).
[Edited on 25/12/2007 by Nicodem]
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
In fact an example of the preparation of a 4-m4thyl-2-aminothiazole is given in Vogel 3rd edition and retained in 5th edition. By substituting the
halogenated MEK (3-chloro-2-butanone) for chloroacetone, Vogel's procedure can serve as a basis for this. Nicodem is quite correct. The amino group
can be diazotized and replaced with hydrogen.
This is a perfectly sensible alternative to proceeding via the oxazole, which anyway requires the same a-haloketone.
It begs the question of whether thiourea is easier to get than formamide, but, it does have the virtue of not requiring P4S10 or Lawsson's reagent.
Nicodem, I'd love to have that thiazole ebook. Where can it be obtained?
Sic gorgeamus a los subjectatus nunc.
|
|
|
JohnWW
International Hazard
    
Posts: 2849
Registered: 27-7-2004
Location: New Zealand
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by kclo4
(cut)When i do this, i want to try to fight most of the seven causes of aging. They are:
1. Cell loss or atrophy
2. Nuclear mutations
3. Mutant mitochondria
4. Cellular senescence
5. Extracellular cross-links
6. Junk outside cells
7. Junk inside cells
http://en.wikipedia.org/wiki/SENS(cut) |
The "key" to preventing ageing, more than anything else, is preventing the non-replacement of cells (maximum life about 7 years, but most live much
shorter lives) as they die. Replacement of cells is by division, which can be prevented or hindered by cross-links developing between protein and in
particular DNA strands, and by mutations, which are caused principally by free-radical damage (which can to a large extent be prevented e.g. by
consumption of anti-oxidants, along with adequate general nutrition); - but the leading systematic cause of cell non-replacement is the shortening of
the telomeres on the ends of each DNA strand.
This shortening of the telomeres is progressive, with each cell division resulting in some loss of length of the telomeres, until they are gone, at
which the cell can no longer divide. The only exceptions are reproductive cells (ova and sperm), and cancer cells, in which a 'switch" in turned on
resulting in the secretion of the enzyme telomerase, which repairs the loss of length of telomeres in cell division. As the result, cancer cells are
effectively "immortal", and in older individuals especially divide at a much faster rate than normal cells. When one is conceived, through creation of
a new diploid cell containing new DNA, the telomeres are at maximum length. There are, no doubt, circumstances that result in greater or lesser rates
of loss of length of telomeres on cell division.
The secret of immortality, therefore, lies primarily in inducing ordinary cells (besides reproductive and cancer cells) to secrete telomerase, to
ensure that the telomeres of new cells are continually repaired. One firm that is doing research to this end is the Geron Corp. Inc. of California; as
I remember their website is www.geron.com or similar.
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
No one has mentioned the relationship between the thiazole ring and the vitamine, thiamin.
The reason that so many substituted thiazoles are flavor chemicals is that the same volatile compounds are released when various animal and vegetable
matter is cooked.
4,5-dimethylthiazole is one such flavor chemical as a googling or an ACS journal search quickly reveals.
These aromas are produced by the thermolysis of thiamin in the proteins.
I am not suggesting the thermolysis of vitamin B1 as a preparative route, although I do happen to have a Kg of thiamin on hand from La Roche. I bought
it to feed to yeast.
I just wanted to point out that thiazoles are our friends (at least sometimes) and so the structure of the ALT series of AGE crosslink breakers is
really not very peculiar.
4,5-dimethylthiazole can be had for as little as $6 a gram (Acros). Aldrich about double that. In both cases, packaging 5 g.
Sic gorgeamus a los subjectatus nunc.
|
|
|
Ozone
International Hazard
    
Posts: 1269
Registered: 28-7-2005
Location: Good Olde USA
Member Is Offline
Mood: Integrated
|
|
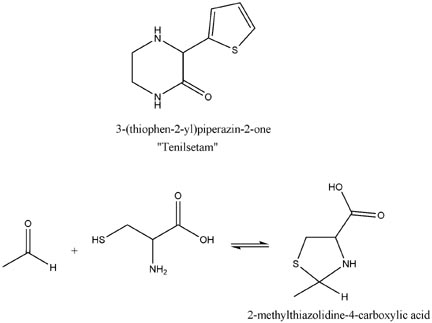
Also of interest would be cysteine (and N-Acetylcysteine). Also consider the reaction of cysteine and aldehydes to yield, for acetaldehyde,
2-methylthiazolidine-4-carboxylic acid. Just a thought, but it looked, at first glance, like it might have some potential as a synthetic route.
Of further interest is:
Recent Advances in the Preparation of Heterocycles on Solid Support: A Review of the Literature by Robert G. Franzén, which is given here:
http://pubs.acs.org/cgi-bin/jtextd?jcchff/2/3/html/cc000002f...
For fun I have attached the structure of tenilsetam (which I think is quite promising) and the potential rxn as previously described.
Merry Christmas,
O3
-Anyone who never made a mistake never tried anything new.
--Albert Einstein
|
|
|
kclo4
National Hazard
   
Posts: 916
Registered: 11-12-2004
Location:
Member Is Offline
Mood: No Mood
|
|
well this is totally off topic of the Alagebrium, but in some ways it is a related compound.
Plus it has been mentioned a couple of times. Aminoguanidine
Aminoguanidine can be made from Nitroguanidine, that being easily made from Guanidine Nitrate. OK so how do we make Guanidine? I UTFSE but i can't
find anything that doesn't seem to have crazy low yields, or use some fancy crap haha
Anyways your saying thiamin is decomposed into 4,5-dimethylthiazole? What els?
I think that seems like a possible way. I really don't like buying things off the internet and pills are easy to get 
[Edited on 28-12-2007 by kclo4]
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
Checking my notes, but not yet UTFSE, I get the following:
Rosco Bodine did some experiments converting calcium cyanurate, from cyanuric acid (pool/spa), to calcium cyanamide. I think there's a related route
that starts with urea.
Axt did a write-up on Nitroguanidine from Sulphamic acid and Urea, that may be in prepub.
All those starting materials are fairly readily available.
I've also got a note on a report of the formation "4, 5-dimethylthiazole by heating D-glucose with hydrogen sulfide and ammonia", but as with many
Maillard type reactions the yields are low and/or a complex mixture is formed. No further reference than that right now.
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
Aminoguanidine, besides being commercially available, can be prepared by means other than reduction of nitroguanidine.
The route I selected and described elsewhere on this forum is the reaction of S-methyl isothiouronium salt with a relatively low concentration of
hydrazine hydrate, this gives a high yield. The S-methyl isothiouronium salt is a commercial product. You could make your own easily enough from
thiourea and an alkylating agent like dimethyl sulfate or iodomethane, but I prefer buying it.
If you want to go the nitroguanidine route I suggest you investigate the purchase of guanidine carbonate, which is readily available and inexpensive.
As the salt of a weak acid it is readily converted to guanidine nitrate and then dehydrate to nitroguanidine, then reduced. Frankly, this is the way
that Axt actually does it (he told me so). I bought a Kg of guanidine carbonate for this purpose, it is sitting not ten feet from where I am right
now.
Once again, rigorous purification is in order, HPLC being the most expedient. Otherwise you will poison your mice forthwith. Ozone is correct about
the dosages. I take guanylguanidine (Glucophage) and consume 2 x 500 mg every day. That's 364 grams a year. I piss like a bat. No, not hanging upside
down!
Incidentally I just ordered Metzger's "Thiazole and its Derivatives" ($35) and will scan it and ost it as pdf. A good way to test my new Microtek book
scanner, which will arrive about the same time. I could not find ebook Nicodem was talking about.
[Edited on 26-12-2007 by Sauron]
Sic gorgeamus a los subjectatus nunc.
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
Sorry for the double post. Returning to the topic of 4,5-dimethylthiazole, here is a related but attractive alternative preparation details in JACS
74, 1719-1720 (1952)
3-chloro-2-butanone (same starting material as usual) is treated with aqueous NaSCN which may be easier to obtain than thiourea (and is markedly less
toxic.) The resulting red oil (after removal of the aqueous layer, and stripping off unreacted 3-chloro-2-butanone and moisture at 80 C and 30mm, is
distilled in vacuo to a pale yellow oil, then distilled again to analytical purity. 3-thiocyano-2-butanone.
This compound reacts with ammonium chloride to give the 2-amino-4,5-dimethylthiazole as in the case of the reaction of the a-chloro ketone with
thiourea. This is then diazotized and reduced to the desired unsubstituted dimethylthiazole.
Likwise 2-hydroxy and 2-mercapto-4,5-dimethylthiazoles are prepared by reactiong the thiocyano compound with water and conc HCl, or with H2S
respectively.
The JACS article is attached along with a trivial correction and a followup article.
I believe the 3-chloro-2-butanone is commercially available. Thus the tedious chlorination of MEK and seperation from the other isomer is avoided.
[Edited on 27-12-2007 by Sauron]
Attachment: ja01127a032.pdf (755kB)
This file has been downloaded 1087 times
Sic gorgeamus a los subjectatus nunc.
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
I have obtained "Thiazole and Its Derivatives" by Metzger, published by Wiley, Parts 1 and 3 (that is, two complete books) from Volume 34 of the
"Chemistry of Heterocyclic Compounds" series.
I am now scanning Part 1 Chapter II, General Synthetic Methods for Thiazoles and Thiazolium Salts"
This chapter is about 170 pages, the first ten pages are attached including the TOC for the chapter, which provides a preliminary overview to anyone
interested in this medicinally and industrially important ring system.
[Edited on 14-4-2008 by Sauron]
Attachment: thiazoles.pdf (300kB)
This file has been downloaded 8641 times
Sic gorgeamus a los subjectatus nunc.
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Thiazole and Its Derivatives was already scanned. I uploaded it in References right now.
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
Thanks, that's a nice surprise. Saves me a lot of effort
[Edited on 14-4-2008 by Sauron]
Sic gorgeamus a los subjectatus nunc.
|
|
|