Aqua_Fortis_100%
Hazard to Others
  
Posts: 302
Registered: 24-12-2006
Location: Brazil
Member Is Offline
Mood: †
|
|
Acetone directly from ethanol
Hi all.
Some hours ago I came with a interesting link, where the author deal with acetone production from ethanol , through zinc/calcium oxide catalyst :
http://cat.inist.fr/?aModele=afficheN&cpsidt=6762883
I tried to find som patent to see a more thorough and explained process..But I can't find any(at least searching briefly english patents at google)!
This is interesting because *seem* to be easy to reproduce at home..
Can anyone talk about tips or then comment about this process and say if is or isn't too easy to make?
thanks!
"The secret of freedom lies in educating people, whereas the secret of tyranny is in keeping them ignorant."
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
It doesn't seem to be in use, although most of the research is fairly new, much since 200 in regards to bioethanol. The same group published a
similar article, but using iron oxide, 5 years later; this suggests that there may be problems with the original.
I don't have online access to the journal, but here's a list starting with your reference
Applied Catalysis
Volume 52, Issue 3, 1 August 1989, Pages 237-248
doi:10.1016/0166-9834(89)80006-5
Applied Catalysis A: General
Volume 172, Issue 1, 24 August 1998, Pages 117-129
doi:10.1016/S0926-860X(98)00106-9
Gas phase conversion of ethanol–water mixtures on copper-based catalysts leads to a significant yield of acetone. Copper catalysts containing
lanthanum oxide stabilized with zirconia, in the form of the so-called La2Zr2O7 pyrochlore compound, exhibit the best performance for the selective
conversion of ethanol into acetone with yields of 96% of the stoichiometric amount at 400°C.
http://www.sciencedirect.com/science?_ob=ArticleURL&_udi...
J. Mater. Chem., 1994, 4, 853 - 858, DOI: 10.1039/JM9940400853
A highly active and highly selective oxide catalyst for the conversion of ethanol to acetone in the presence of water vapour
Tsuyoshi Nakajima, Hisashi Nameta, Shozi Mishima, Isao Matsuzaki and Kozo Tanabe
In the presence of water vapour and a suitable catalyst, ethanol is converted to acetone rather than to ethylene and acetaldehyde. In order to develop
an appropriate catalyst for this reaction, steady-state catalytic activities and selectivities were studied for 24 oxide catalysts. It was found that
the acetone selectivity is high on a catalyst having both surface acidity and basicity, suggesting that the acetone formation is an acid-base
bifunctional catalytic reaction. Iron oxide is superior to the other oxides studied here in both conversion and acetone selectivity. The superiority
is greatly enhanced by mixing iron oxide with zinc oxide
|
|
|
Aqua_Fortis_100%
Hazard to Others
  
Posts: 302
Registered: 24-12-2006
Location: Brazil
Member Is Offline
Mood: †
|
|
| Quote: | Originally posted by not_important:
Gas phase conversion of ethanol–water mixtures on copper-based catalysts leads to a significant yield of acetone. Copper catalysts containing
lanthanum oxide stabilized with zirconia, in the form of the so-called La2Zr2O7 pyrochlore compound, exhibit the best performance for the selective
conversion of ethanol into acetone with yields of 96% of the stoichiometric amount at 400°C.
|
I'm confused. Why copper based catalysts in this study produces acetone rather than acetaldehyde/acetic acid (maybe because of the other compounds
present in the catalyst?)??
The second paper you provided , it mentions that iron oxide is also used.. This is interesting.. So mixing iron oxide and zinc oxide , putting the
mix in a iron tube , heating it and passing ethanol/water vapor will yield acetone (???).. If yes, what the best temp. to catalyst do its job and what
is the ratio water/ethanol , how to make the catalyst (of course, if all this is really feasible at home)???
Thanks!
"The secret of freedom lies in educating people, whereas the secret of tyranny is in keeping them ignorant."
|
|
|
gregxy
Hazard to Others
  
Posts: 421
Registered: 26-5-2006
Member Is Offline
Mood: No Mood
|
|
Given that ethanol has 2 carbons and acetone 3 it does not seem possible (or if it is it must be a complex mechanism). It is probably a misprint,
maybe from isopropyl alcohol?
Around here it is easier to get acetone than ethanol.
|
|
|
MagicJigPipe
International Hazard
    
Posts: 1554
Registered: 19-9-2007
Location: USA
Member Is Offline
Mood: Suspicious
|
|
You are totallt right. It would take 1 1/3 ethanol to get the required carbon. Not likely.
"There must be no barriers to freedom of inquiry ... There is no place for dogma in science. The scientist is free, and must be free to ask any
question, to doubt any assertion, to seek for any evidence, to correct any errors. ... We know that the only way to avoid error is to detect it and
that the only way to detect it is to be free to inquire. And we know that as long as men are free to ask what they must, free to say what they think,
free to think what they will, freedom can never be lost, and science can never regress." -J. Robert Oppenheimer
|
|
|
arevelacao
Harmless

Posts: 9
Registered: 4-9-2006
Member Is Offline
Mood: No Mood
|
|
An aldol condensation that lead to the formation of acetone from ethanol (via acetaldehyde). The catalysts are frequently basic oxides.
|
|
|
MagicJigPipe
International Hazard
    
Posts: 1554
Registered: 19-9-2007
Location: USA
Member Is Offline
Mood: Suspicious
|
|
Now that makes more sense. Durrr Me!!!
"There must be no barriers to freedom of inquiry ... There is no place for dogma in science. The scientist is free, and must be free to ask any
question, to doubt any assertion, to seek for any evidence, to correct any errors. ... We know that the only way to avoid error is to detect it and
that the only way to detect it is to be free to inquire. And we know that as long as men are free to ask what they must, free to say what they think,
free to think what they will, freedom can never be lost, and science can never regress." -J. Robert Oppenheimer
|
|
|
Xenoid
National Hazard
   
Posts: 775
Registered: 14-6-2007
Location: Springs Junction, New Zealand
Member Is Offline
Mood: Comfortably Numb
|
|
| Quote: | Originally posted by MagicJigPipe
You are totallt right. It would take 1 1/3 ethanol to get the required carbon. Not likely. |
| Quote: |
Now that makes more sense. Durrr Me!!!
|
Durrr You, indeed! Another two useful contributions.....
Aqua_Fortis_100% and not_important quoted 3 respectable journals, why would you claim to know better! Are you a Professor of Organic Chemistry?
Methinks you are suffering from the dreaded "overposting syndrome"!.... 
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
Note that the member interested in this lives in Brazil, and to paraphrase an moderately old song "They've got a zillion tons of ethanol in Brazil"
Looks like we'll need to request those journal articles, as I'd just be handwaving as to what reactions are occurring, the conditions needed, what
catalysts are best and why.
|
|
|
Aqua_Fortis_100%
Hazard to Others
  
Posts: 302
Registered: 24-12-2006
Location: Brazil
Member Is Offline
Mood: †
|
|
Yeah ! not_important said all! Ethyl alcohol is cheap.
To say more 'old song'  : Pure acetone , AFAIK , is only otc in USA and some
other very few and happy countries.. and purification of nail poilsh remover is a pain. : Pure acetone , AFAIK , is only otc in USA and some
other very few and happy countries.. and purification of nail poilsh remover is a pain.
I have some homemade zinc basic carbonate on hand.. Can I expect some sucess mixing this with CaCO3 and/or FeCO3 and heating the mix until all
decompose ?
This other paper shows the abstract using CaCO3/zinc oxide:
http://sciencelinks.jp/j-east/article/200018/000020001800A06...
not_important, these journal articles are somewhat hard to non registered people (as I.. $$$$) to get it..I also found an Brazilian article :
http://www.biblioteca.ufpb.br/catalogo_96_2000/campus2/eng_q... (the second paper.. I'm trying to acess this paper but they still not reply the
email I sent yesterday)
Are there some patents describing or at least mentioning this process??
[Edited on 5-1-2008 by Aqua_Fortis_100%]
"The secret of freedom lies in educating people, whereas the secret of tyranny is in keeping them ignorant."
|
|
|
MagicJigPipe
International Hazard
    
Posts: 1554
Registered: 19-9-2007
Location: USA
Member Is Offline
Mood: Suspicious
|
|
Yes, I am. What's the prescription?
"There must be no barriers to freedom of inquiry ... There is no place for dogma in science. The scientist is free, and must be free to ask any
question, to doubt any assertion, to seek for any evidence, to correct any errors. ... We know that the only way to avoid error is to detect it and
that the only way to detect it is to be free to inquire. And we know that as long as men are free to ask what they must, free to say what they think,
free to think what they will, freedom can never be lost, and science can never regress." -J. Robert Oppenheimer
|
|
|
gregxy
Hazard to Others
  
Posts: 421
Registered: 26-5-2006
Member Is Offline
Mood: No Mood
|
|
An aldol condensation of acetaldhyde + ethanol would give
MEK not acetone.
The one reference says oxidation to acetate. Then thermal decomposition of 2 acetate gives acetone + CO2. So this must be how it works.
Maybe it would be easier to ferment ethanol to acetic acid,
neutralize this with baking soda, dry it out and then get
acetone by heating the sodium acetate?
|
|
|
Aqua_Fortis_100%
Hazard to Others
  
Posts: 302
Registered: 24-12-2006
Location: Brazil
Member Is Offline
Mood: †
|
|
gregxy I dont think it works like an aldol condensation , but rather than the oxidation of EtOH with air to acetic which reaches the calcium
oxide/carbonate and forms calcium acetate that way the thermal decomposition cause it to forms almost as quick as it was formed acetone and the
calcium carbonate forms again (over and over again as it must be a good catalyst..I think that the 'trick' maybe is the correct control of the
temperature to this catalyst work properly, because one time I see in frogfot's page that the Piria method for producing acetone will produce at the
bottom of the vessel a 'char-like' CaCO3...I still dont know why the zinc oxide, but can anyone says that it works like calcium carbonate/oxide??)
And I'm doubt that sodium acetate will produce reasonable amounts of acetone.. (well, what I learned is that only +2 cations attached to acetate can
drive this reaction, like Ca++, Ba++, etc..But , again, I can be wrong)
"The secret of freedom lies in educating people, whereas the secret of tyranny is in keeping them ignorant."
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
If acetaldehyde + ethanol -> MEK then why not
formaldehyde + ethanol -> acetone
or
acetaldehyde + methanol
or
acetaldehyde + formaldehyde
Personally I would rather have a process to make ethanol from acetone than vice versa.
I am not going to propose any mechanism for the above pairs of compounds.
As forthermolysis of acetic acid, clearly they are talking about generation of ketene and somehow obtaining acetone from that. Not a home lab
procedure IMO.
Sic gorgeamus a los subjectatus nunc.
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
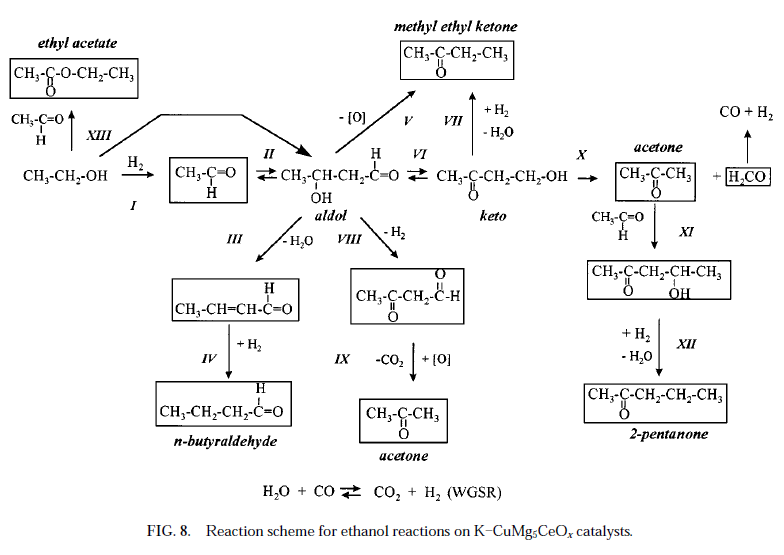
From this publicly available document http://iglesia.cchem.berkeley.edu/JournalofCatalysis_171_130...
Isobutanol and Methanol Synthesis on Copper Catalysts Supported on Modified Magnesium Oxide, comes the attached image of a set of reactions
as part of the study on alcohol coupling reaction pathways .
Rather messy, unless a catalyst is very specific to one product or another, although all the products could be useful.
[Edited on 6-1-2008 by not_important]
|
|
|
arevelacao
Harmless

Posts: 9
Registered: 4-9-2006
Member Is Offline
Mood: No Mood
|
|
Oh, an aldon condensation happens after a dehydrogenation of ethanol to acetaldehyde, it depends directly on catalyst surface and the temperature.
2CH3CH2OH -----> CH3COCH3 + CO + 3H2
This decomposition is basead in ZnO-catalysts, and the temperature about 670K.
As seen, this is a process to production of H2 via ethanol. I don't really know, over others catalysts, how is performed the specific reactions.
This is an example:
Direct production of hydrogen from ethanolic aqueous solutions over oxide
Jordi Llorca, Pilar Ramírez de la Piscina, Joaquim Sales and Narcís Homs
Departament de Química Inorgànica, Facultat de Química, Universitat de Barcelona,
Steam-reforming of ethanol over ZnO gives highly effective
production of CO-free H2: 5.1 mol of H2 per mol of reacted
ethanol is formed at 723 K under 100% ethanol conver-sion.
The following oxides with the indicated BET surface area were used: MgO (prepared by adding ammonia to a MgCl2 solution, 110m2 g-1), g-Al2O3 (Girdler,
188m2 g-1), SiO2 (Degussa-Hüls, 200m2 g-1), TiO2 (Degussa-Hüls, 45m2 g-1), V2O5 (Merck, 22m2 g-1), ZnO (1) (Asturienne New Jersey, 11m2 g-1), ZnO
(2) (prepared by decomposition of 3ZnO·2Zn-CO3·3H2O, 100m2 g-1), La2O3 (Merck, 11m2 g-1), CeO2 (Aldrich, 17m2 g-1), Sm2O3 (Merck, 9m2 g-1).
Steam-reforming of ethanol was carried out between 573 and 723K, at atmospheric pressure, using a 1:13:70 C2H5OH:H2O:Ar stream (molar ratio) and 0.1g
of the appropriate oxide diluted with inactive SiC, under a gas hourly space velocity (GHSV) of 5000h-1 . After periods of 2 h at each temperature,
the temperature was increased consecutively from 573 to 623, 673 and 723K; at the final temperature the reaction was conducted over a period of 20 h.
Products were analysed on-line by gas chromatography. Hydrogen was analysed with a TCD using Ar as a carrier gas, CO and CO2 were analysed with an FID
after passing through a methanizer, and hydrocarbons as well as oxygenated products were separated with a capillary column and analysed with an FID.
http://rapidshare.com/files/81752383/Direct_production_of_hy...
|
|
|
YT2095
International Hazard
    
Posts: 1091
Registered: 31-5-2003
Location: Just left of Europe and down a bit.
Member Is Offline
Mood: within Nominal Parameters
|
|
| Quote: | Originally posted by MagicJigPipe
Yes, I am. What's the prescription? |
a Change of Attitude would be a Good start!
things like: "Mood: Xenoid is a silly goose."
doesn`t bode well here (or anywhere for that matter, you`re Already being Watched over at SFN too.
you would do Well to keep your mouth shut and Learn from these people, here AND there!
\"In a world full of wonders mankind has managed to invent boredom\" - Death
Twinkies don\'t have a shelf life. They have a half-life! -Caine (a friend of mine)
|
|
|
gregxy
Hazard to Others
  
Posts: 421
Registered: 26-5-2006
Member Is Offline
Mood: No Mood
|
|
My memory is bad, I thought an aldol condensation was
between an aldehyde and alcohol to form a ketone.
However according to Wikipedia it is a reaction between an aldehyde and a ketone to form a new ketone.
My point however is that the aldol reaction does not liberate CO2 and in this case can only produce products with an even number of carbon atoms.
Anyway it looks like this process without careful control of
the reaction conditions, is going to produce a
wide range of products that may be difficult to separate.
It might be easier to distil the acetone from nail polish
remover. (But then I can buy a liter of acetone at the
hard ware store)
Aqua_fortis you are probably correct, sodium acetate would
probably not work, calcium would be needed, but
alcohol -> acetic acid -> calcium acetate -> acetone
seems like it might be easier for the home chemist.
|
|
|