| Pages:
1
..
11
12
13 |
dann2
International Hazard
    
Posts: 1523
Registered: 31-1-2007
Member Is Offline
Mood: No Mood
|
|
Hello Xenoid,
"Whilst not wanting to return to the heady days of early '08,"
That's a pity. I am due my yearly (self administered) allocation of SCIENCE MADNESS 
Congradulations for starters.
I cannot find a figure of the size (surface area) of the Anode. How big is it?
Do you think the Perchlorate runs lowered the Chlorate to a low level. Did (or would) you get a 'yogurt ppt' if you added KCl to the Sodium
Perchlorate cell?
I presume you did not attempt to pH controll any of the cells? (except the time you added acid at the start)
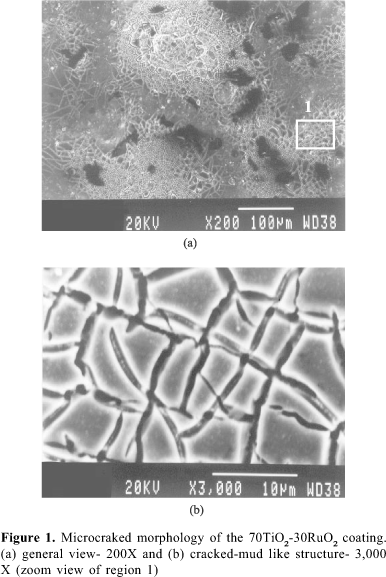
The 'cracked mud morphology' (not to mention similar crystal structure as you said)of MMO always looks like 'good stuff' for adding coatings onto.
Cheers,
Dann2
|
|
|
Xenoid
National Hazard
   
Posts: 775
Registered: 14-6-2007
Location: Springs Junction, New Zealand
Member Is Offline
Mood: Comfortably Numb
|
|
Hi dann2 - Wow, I wouldn't have thought MMO looked like that! Yes, the MnO2 bonds really well, with my earlier '08 anodes, I actually "polished" off
the crusty build-up because I thought it was a bit "soft". That was a mistake, just let it build-up and it gets quite thick, quickly.
I did some other runs, not reported in the earlier post, and I have ended up with about 8 soft drink bottles with assorted liquors in them. Some went
in more than 1 cell. I haven't extracted any KClO4 from the potassium runs, there doesn't appear to be much there, I just recovered the chlorate. I
extracted about a 100g of KClO4 from one of the sodium runs, using metathesis. My son has already used most of this in "flash powder". The MnO2
contamination is a bit persistant, if you want pure white product you'll need multiple crystallisations. I've just recovered chlorate from a couple of
runs, perchlorate wasn't high. There is still quite a bit of liquor to process, if I ever get around to it.
It would be nice to see someone else make some perchlorate using MnO2/MMO. Despite what I said a few years ago, MnO2 anodes are incredibly cheap and
easy to experiment with over MMO. Just spray on a few more layers of another doping element and away you go!
I was disappointed that the Bi doping (1.3% and 3.9%) didn't seem to have any effect, maybe Sb or As are the way to go!
BTW the electrodes are 18cm x 5cm. It is almost impossible to estimate the surface area of this mesh, so I don't know what current densities I was
using!
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
Did you try combined Co and Bi doping of the MnO2, tested with continuing the electrolysis to a point of test for percholorate? Have you tried the
addition of SnO2 in the above scheme, or Bi doped SnO2 applied to the MMO ?
|
|
|
Xenoid
National Hazard
   
Posts: 775
Registered: 14-6-2007
Location: Springs Junction, New Zealand
Member Is Offline
Mood: Comfortably Numb
|
|
Geez - Rosco, there is a lot of work involved in all this, the trouble is one ends up with numerous bottles of half converted chlorate liquors, etc.
There is a lot of chemicals involved because of the saturated solutions, and a lot of recrystallising to get them back again!
I was going to check out tin and antimony but I ran into the problem I had before, what to use as a combustible dopant. I made some tin citrate
complex (which is interesting in itself, viscous, glassy) but when heated, it swells up like a Pharaoh's Serpent.
I'll leave further investigations to someone else.
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
I read your earlier posts a couple of times and your odd conclusion about the bismuth doping not seeming to have any effect on the MnO2 is opposite
the conclusion which I got from reading your description of the experiments. The impression I got was that the cobalt doping and the bismuth doping
both individually helped the MnO2, and would probably work better together in combination. I was also thinking that SnO2 would likely benefit the mix
further.
As a different strategy entirely, I still think the Bi doped SnO2 described in US6777477 applied to MMO would seem to be most likely as the simplest
and shortest path for making a commercial MMO anode have selectivity for perchlorate
and would be a baked coating alternative to electrodeposited PbO2. I continue accumulating materials needed to test these ideas and many others also
which I have stated could be worthy experiments, if indeed such experiments must await my own investigation, which may be awhile because I have quite
a backlog of such experiments. Searching the literature for any information of potential value has been pretty extensive and thorough. So these ideas
involving the two schemes which may enhance the MMO to make it selective for perchlorate do track with what that search of the literature suggests or
by inference indicates would probably work, for whoever first investigates to see  Last one in is a rotten egg Last one in is a rotten egg 
Oh it's such a chore, doing experiments ...like peelin' taters 
http://www.youtube.com/watch?v=r91Iha3tc80&fmt=18
[Edited on 15-3-2010 by Rosco Bodine]
|
|
|
dann2
International Hazard
    
Posts: 1523
Registered: 31-1-2007
Member Is Offline
Mood: No Mood
|
|
Hello,
Baked on Tin Oxide has a similar morphology. The notion of needing a good 'seal' on Titanium is a misnomer. It's a Valve metal after all!
I intend to try MnO2 onto etched Ti as there are lots of patents that use MnO2 on bare Ti. It looks like pH controll will not help with the pink
discolouration (coat wearing) since you got a pink colour after 15 minutes in the cell with acid added at the start. But is was a K cell, perhaps Na
will be kinder????????????/
If I were to guess that the Anodes are 70% of 18 x 5 x 2 sides = 190 square cm
Thats a CD of about 64mA per square cm.
SnO2 coatings onto bare Ti are a piece of piss when you have SnCl4:5H20 but SnCl4:5H2O is not OTC or easy to make. The Tin Citrate may be the way to
go (perhaps more OTC).
Tin Oxide Anodes are TOUGH IMO. I ran an Sb doped one in a pure (no Chloride) Na Perchlorate cell for approx. a forthnight, 4 time the 100% CE 'run
time'. It made fair amounts of Perchlorate, I could get a yogurt PPT and it stood up well but it did not seem to be able to take the Chlorate
concentration to low levels (LD is great for that). 69% of extracted stuff was Chlorate, 31% Perk.:
http://www.oxidizing.110mb.com/chlorate/semi.html
With Bi doping (as per a blow by blow account in a patent) perhaps there is a workable Anode.
@ Xenoid. Don't stop now, when you are just coming to the beach (after a long swim), feet almost touching the bottom...... 
NOT HAVING ENOUGH TIME IS SIMPLY NOT A GOOD ENOUGH EXCUSE (it is for some though  ). To save time you could always stick a broom up you ass and sweep the floors as you go about you Anode making
tasks................. ). To save time you could always stick a broom up you ass and sweep the floors as you go about you Anode making
tasks.................
Dann2
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
When it comes to anything going onto bareass Ti, a conductive interface layer is probably essential as the first order of business, on a preferably
oxalic acid etched
CP grade of Ti, either making the conductive interface using the Ru doping of MMO fame, or using Co or mixed Co - Ni spinel as the substitute.
Adding a DTO baked coating over that Ti substrate which has been thus rendered "anodically conductive" via MMO or spinel strategy, the substrate is
sealed and ready to receive the catalytic working coating which has a high oxygen overvoltage and
selectivity for perchlorate. If Sb DTO will make perchlorate at all, then Bi DTO
should work ten times better, since the Bi is reportedly selective for perchlorate.
But the real secret is the polka dot anode configuration where the finished Bi DTO baked anode is sandwiched in a perforated plexiglass mask and
speckled with electrodeposited alpha PbO2 islands resulting in a bi-electrode working surface,
producing wheelbarrow fulls of perchorate from ions who surrender and be
whatever they are wanted to become, rather than be tormented by any more confusing anode polkas.
http://www.youtube.com/watch?v=9xAi80ShG0I&fmt=18 Yakety Yak
[Edited on 15-3-2010 by Rosco Bodine]
|
|
|
avi66
Harmless

Posts: 46
Registered: 8-4-2010
Member Is Offline
Mood: No Mood
|
|
i try to make mno2 anode from a titanium strip, i already have CoO but because cobalt salts are carcinogenic im looking for another non-toxic
alternative layer to put between ti and mno2, can i make sncl:5h2o/h2o 50:50 solution and burn it over my ti strip, to get sno2 layer which allow me
to put on her mno2 ? if someone know alternative non-toxic interface layer please post it.
|
|
|
mnick12
Hazard to Others
  
Posts: 404
Registered: 30-12-2009
Location: In the lab w/ Dr. Evil
Member Is Offline
Mood: devious
|
|
Hate to bump an old thread, but I think this is the most appropriate thread to ask my question.
About a year ago I had some success using a cobalt oxide/manganese dioxide on a titanium sheet in a chlorate cell. Well since then I have not done any
electrochemistry, but now that I have a bit of free time I thought it would be fun to continue where I left off. Anyway the titanium sheet was etched
in hot phosphoric acid to remove the old oxides and provide a clean surface to deposit the new oxides. After etching followed by a quick wash 5 layers
of cobalt oxide were deposited onto the sheet from a 20% cobalt nitrate solution. The loose oxide was removed by wipping the anode with a damp towel.
Following this a layer of electrolytic MnO2 was deposited using anodic oxiation of MnSO4, this layer of MnO2 is quite durable and looks very cool
(reflective black almost like graphite). Anyway that is where I left off and I probably wont be able to do anything for at least a week. My question
is has anyone had any expirience with electrodeposited MnO2? And if so how does it compare to the thermal decomposition method.
Oh and my plans are for a sodium chlorate cell.
Thanks!
|
|
|
Xenoid
National Hazard
   
Posts: 775
Registered: 14-6-2007
Location: Springs Junction, New Zealand
Member Is Offline
Mood: Comfortably Numb
|
|
Hi mnick12
I only ever tried electrodeposited MnO2 on carbon rods in my early experiments, it did not bond well and flaked off. There are plenty of mentions of
electrodeposited MnO2 anodes in the patents. Most use hot, >90 oC. acidic solutions. Did you use a hot solution?
In retrospect, i guess it may have been easier to go the electrodeposited route rather than applying all the multiple coats of thermal MnO2.
Try using an electrodeposited MnO2 anode to make perchlorate from a sodium chlorate solution (not potassium chlorate solution).
|
|
|
mnick12
Hazard to Others
  
Posts: 404
Registered: 30-12-2009
Location: In the lab w/ Dr. Evil
Member Is Offline
Mood: devious
|
|
Hi Xenoid,
The MnO2 was deposited by using a modified version of Nurdrages procedure. From his video he mentions problems with flaking of the new oxide layer, my
guess is to high of a current density and to concentrated of a solution. So for my procedure I used 2l of dH2O 180g of MnSO4 and 50ml of conc H2SO4.
No external heating was applied so the solution was around 30C when I began plating. I forget the size of my anode so I do not know what the current
density was, but I was drawing ~1amp 5-6V. Plating took about 10 minutes to complete, and my thought is if this oxide layer flakes off like Nurdrages,
I will re-deposit the MnO2 and bake the anode @375C for a few hrs and see if that helps.
What current density would you recomend for an anode of this type?
My end game in this endeavour is sodium chlorate for some organic oxidations, however it would be neet if this anode could make perchlorates.
|
|
|
Xenoid
National Hazard
   
Posts: 775
Registered: 14-6-2007
Location: Springs Junction, New Zealand
Member Is Offline
Mood: Comfortably Numb
|
|
Hi mnick12
A quick perusal of my "patents folder" reveals US Patents 4028215 and 4265728, among others, describe electro-deposited MnO2 electrodes.
MnO2 is usually electro-deposited in 150g/L MnSO4 solutions with 25g/L concentrated H2SO4 and at temperatures of 80 - 100 oC. Mn(NO3)2 solutions are
also used. Low current densities seem to give the best results, 1 to 4 mA/cm^2
You will need to bake the electrode at ~400 oC. for about 30 mins. to convert the MnO2 to the more desirable beta form (rutile structure - from
memory). Thermally applied tin dioxide seems to be a preferred interface coating.
|
|
|
mnick12
Hazard to Others
  
Posts: 404
Registered: 30-12-2009
Location: In the lab w/ Dr. Evil
Member Is Offline
Mood: devious
|
|
Hmm I dont have any tin compounds however I suppose I could make some from some tine wire.
I know the gamma form is what results from most electrodeposition, but why is the beta form more desirable? More durable, better conductivity? Also on
a slightly different note, why not electroplate cobalt oxides from solution. I cant comment on cylndrical titanium anodes, but as far as sheets go it
is quite difficult to thermally decompose an even layer of cobalt oxide. It seems that the electrodeposition cotes the anode very evenly, and this I
imagine would be very desirable under anodic conditions.
Oh and thank you for those patents.
|
|
|
Xenoid
National Hazard
   
Posts: 775
Registered: 14-6-2007
Location: Springs Junction, New Zealand
Member Is Offline
Mood: Comfortably Numb
|
|
Quote: Originally posted by mnick12  | | ... but why is the beta form more desirable? More durable, better conductivity? Also on a slightly different note, why not electroplate cobalt oxides
from solution. I cant comment on cylndrical titanium anodes, but as far as sheets go it is quite difficult to thermally decompose an even layer of
cobalt oxide. It seems that the electrodeposition cotes the anode very evenly, and this I imagine would be very desirable under anodic conditions.
|
More catalytically active, especially in perchlorate production.
"Raw" titanium will passivate (anodize) and become non-conducting without the interface coat. The reason I mention tin dioxide rather than cobalt
oxide, is that it also has a "rutile" structure and could possibly bond well with beta-MnO2.
Your experience with electrodeposition would appear to be at odds with most people. Nodules forming on PbO2 anodes being a particular problem (see
dann2 and Swede's efforts). Although a thin coat may appear smooth it may be necessary to allow the thickness to increase to create a reliable and
long-lasting anode.
Good luck with your experiments, keep us informed (a few images).
I may have a go at this myself when I finish the current round of house renovating 
|
|
|
mnick12
Hazard to Others
  
Posts: 404
Registered: 30-12-2009
Location: In the lab w/ Dr. Evil
Member Is Offline
Mood: devious
|
|
Quote: Originally posted by Xenoid  | Your experience with electrodeposition would appear to be at odds with most people. Nodules forming on PbO2 anodes being a particular problem (see
dann2 and Swede's efforts). Although a thin coat may appear smooth it may be necessary to allow the thickness to increase to create a reliable and
long-lasting anode.
Good luck with your experiments, keep us informed (a few images).
I may have a go at this myself when I finish the current round of house renovating  |
I imagine a thicker coating would be a bit more uneven, but not to the same extent as some of those lead dioxide anodes as the anodes surface is much
more even than those MMO mesh. But before I go any thicker on the electrodepostited MnO2 I want to see how what I have now performs. Tomorrow I will
go ahead and bake the current MnO2 layer for a while at 400C and post a few pictures. However before I get test the anode I need to rewind my HID
ballast as a power supply.
|
|
|
mnick12
Hazard to Others
  
Posts: 404
Registered: 30-12-2009
Location: In the lab w/ Dr. Evil
Member Is Offline
Mood: devious
|
|
Well things arent looking so well,
Today I took the MnO2 and subjected it to 400C on my hotplate for 1hr in hopes of converting the gamma-MnO2 into the more desirable beta-MnO2. like I
said in a previous post the electrolytic MnO2 is has pretty good mechanical strength and cannot be removed by rubbing with a piece of cloth. However
after baking for 1 hr and cooling the MnO2 has begun to flake off, it is not that noticeable in pictures however in real life it is easy to see. It
looks much like dried mud, in that it curls up forming small flakes which are easily removed. I will have to get back to this later, but I think the
next time I will put a tin oxide interface between the two layers.
Anyway here a few pics, not really that exciting but better than nothing.
This is the anode prior to baking, notice how reflective it is and the interface between the cobalt and manganese oxides (shown by that chop-stick)

And this is post bake, like I said the flaking is not seen through this picture but it is noticeable person.

Anyway more work later, suggestions are welcome.
|
|
|
Xenoid
National Hazard
   
Posts: 775
Registered: 14-6-2007
Location: Springs Junction, New Zealand
Member Is Offline
Mood: Comfortably Numb
|
|
Sorry to see that, mnick12 
I'm not sure I like the idea of baking the anode on a hot plate! Even heating with a hot air gun (if you can get it up to that temperature) would be
more preferable. Differential expansion on that Ti sheet might be a problem, mesh would likely be better in this regard.
The structural differences between the Co3O4 (spinel) and the MnO2 (rutile) is the most likely cause of the flaking, however.
Don't forget the SnO2 needs doping with Sb, there are some techniques on dann2's page:
http://www.oxidizing.110mb.com/chlorate/semi.html
|
|
|
Xenoid
National Hazard
   
Posts: 775
Registered: 14-6-2007
Location: Springs Junction, New Zealand
Member Is Offline
Mood: Comfortably Numb
|
|
mnick12, I came across this paper, which may be of interest!
http://esl.ecsdl.org/content/4/6/D1.full.pdf+html
"Electrochemical synthesis of beta- and gamma- manganese dioxide under hydrothermal conditions"
Essentially, with regard to what you are trying to do, it claims that at 130 oC. and pH=1, deposition of MnO2 is pure beta-MnO2. At lower temperatures
mixed gamma and beta is produced.
They use a fancy autoclave for their experiments, but it may be possible to achieve 130 oC. in a pressure cooker modified for electrodeposition! At
113 oC. a mixture of beta and gamma was produced and at 92 oC. the major phase was gamma.
It may be that electrodeposition at, say, 95 oC. results in enough beta MnO2 to produce a suitable anode without having to resort to further heat
treatment.
They use a 0.3M MnSO4 solution (that's only about 50g/L) and a low current density of 0.36 mA/cm^2
EDIT: Duh...! pH=1. I see why they used a teflon lined autoclave, a domestic pressure cooker isn't going to stand up to those conditions very well
...!
[Edited on 5-8-2012 by Xenoid]
|
|
|
mnick12
Hazard to Others
  
Posts: 404
Registered: 30-12-2009
Location: In the lab w/ Dr. Evil
Member Is Offline
Mood: devious
|
|
Interesting,
Unfortunately I dont have the money for a teflon lined autoclave, and high pressure sulfuric acid does not seem like that much fun. However I imagine
a big beaker (say 4l?) and my hot plate should be able to reach 95C no problem.
Also I may be loosing my mind here, but sulfuric acid solutions with a pH of 1 are quite dilute. IIRC pH is the -log[H+ ions], and the bisulfate
proton is not as acidic as the first, so that would mean a 0.1Molar solution of sulfuric acid would have a pH of around 1. Still though at 95C that
would be nasty stuff.
At this time I do not have a beaker large enough to try this out, but I will order one later. Sadly I do not think I will be able to do much in the
way of extensive testing for at least 4 months since I am going to be out of town for an internship. However when I return I will be able to continue
where I left off.
|
|
|
Boron Trioxide
Harmless

Posts: 42
Registered: 18-6-2012
Member Is Offline
Mood: No Mood
|
|
Hi sorry to bump up this thread, though has anyone determined if the Cobalt interface layer is necessary, or if MnO2 alone will produce
chlorate/perchlorate? The reason I ask is because cobalt compounds are very expensive.
|
|
|
Swede
Hazard to Others
  
Posts: 491
Registered: 4-9-2008
Member Is Offline
Mood: No Mood
|
|
I don't know if Mn will make chlorate, nor am I familiar with cobalt. If you want to make your own MMO, it is probably best to start with some of the
Henri Beer patents that began in the 1960's.
I have been gathering the reagents and a huge binder full of patents in an attempt to replicate some of his work. Unfortunately, the chemicals are
expensive. Fortunately, a little goes a long way. I've stocked up on Ru salts, tin, and some palladium. TiCl3 can be easily made by dissolving Ti
sponge in HCl.

Also bought a little abrasive blaster to prep the Ti:

The goal is ultimately to explore alternate formulations, perhaps something that can produce perchlorate as well as simply chlorate. Also, I want to
explore the technology so as to learn how to lay down a conductive layer for PbO2.
In a nutshell, MMO is made by painting on, dipping, or spraying, an alcoholic mixture of various chlorides of Ti, Ru, Ir, Sn, many others; then
thermally decomposing each coat at about 300 C.
The variations in catalysts are nearly infinite, and I'd like to explore Mn and Co. It would be nice to be able to make effective MMO that actually
works without the expensive Pt group metals, but first things first... got to get the process down before varying it.
|
|
|
| Pages:
1
..
11
12
13 |