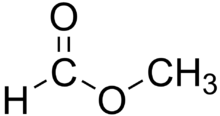
Ester
 |
This article is a stub. Please help Sciencemadness Wiki by expanding it, adding pictures, and improving existing text.
|
In organic chemistry, esters are a group of compounds in which the hydroxyl group of an acid has been replaced by an oxygen atom attached to an alkyl group. Organic esters are often centered around a carbon atom that contains a double bond to an oxygen atom, a single bond to another oxygen atom, and a single bond to a carbon atom. They are often derived from a carboxylic acid and an alcohol, such as in ethyl acetate and methyl salicylate. Many esterifications are very simply done between these components using a dehydrating agent such as sulfuric acid to drive the reaction.
The somewhat misleading term "inorganic ester" refers to esters in which the central oxygen atom is bound to an atom other than carbon that have usually been derived instead from an inorganic acid, such as in trimethyl borate.
Some compounds contain multiple ester groups and are sometimes denoted diesters, triesters, etc. Polyesters are polymers that contain esters as part of their main chain.
Esters are often characterized by a pleasant fruity or solvent-like odor and a fruity taste, as esters are often the source of the familiar smells and tastes of many fruits and botanical extracts, such as wintergreen. This leads them to be frequent candidates for fragrances and flavorings.