Difference between revisions of "Oxalic acid"
| Line 1: | Line 1: | ||
| − | + | {{Chembox | |
| − | [[ | + | | Name =Oxalic acid |
| + | | Reference = | ||
| + | | IUPACName =Oxalic acid | ||
| + | | PIN = | ||
| + | | SystematicName =Ethanedioic acid | ||
| + | | OtherNames = {{Unbulleted list | ||
| + | | ''name1'' | ||
| + | | ''name2'' | ||
| + | ... | ||
| + | | ''name50'' | ||
| + | }} | ||
| + | <!-- Images --> | ||
| + | | ImageFile =Oxalic_acid_dihydrate.jpg | ||
| + | | ImageSize = 250 | ||
| + | | ImageAlt = | ||
| + | | ImageName = Oxalic acid dihydrate | ||
| + | | ImageFile1 = Oxalic_acid.png | ||
| + | | ImageSize1 = | ||
| + | | ImageAlt1 = | ||
| + | | ImageName1 = | ||
| + | | ImageFile2 = | ||
| + | | ImageSize2 = | ||
| + | | ImageAlt2 = | ||
| + | | ImageName2 = | ||
| + | | ImageFile3 = | ||
| + | | ImageSize3 = | ||
| + | | ImageAlt3 = | ||
| + | | ImageName3 = | ||
| + | | ImageFileL1 = | ||
| + | | ImageSizeL1 = | ||
| + | | ImageAltL1 = | ||
| + | | ImageNameL1 = | ||
| + | | ImageFileR1 = | ||
| + | | ImageSizeR1 = | ||
| + | | ImageAltR1 = | ||
| + | | ImageNameR1 = | ||
| + | | ImageFileL2 = | ||
| + | | ImageSizeL2 = | ||
| + | | ImageAltL2 = | ||
| + | | ImageNameL2 = | ||
| + | | ImageFileR2 = | ||
| + | | ImageSizeR2 = | ||
| + | | ImageAltR2 = | ||
| + | | ImageNameR2 = | ||
| + | <!-- Sections --> | ||
| + | | Section1 = {{Chembox Identifiers | ||
| + | | 3DMet = | ||
| + | | Abbreviations = | ||
| + | | SMILES = | ||
| + | }} | ||
| + | | Section2 = {{Chembox Properties | ||
| + | | AtmosphericOHRateConstant = | ||
| + | | Appearance = White crystals | ||
| + | | Odor = Odorless | ||
| + | | BoilingPt = | ||
| + | | BoilingPtC = | ||
| + | | BoilingPt_ref = | ||
| + | | BoilingPt_notes = | ||
| + | | Density = | ||
| + | | Formula = H<sub>2</sub>C<sub>2</sub>O<sub>4</sub> | ||
| + | | HenryConstant = | ||
| + | | LogP = | ||
| + | | MolarMass = 90.035 (anhydrous)<br/ >126.066 (dihydrate) | ||
| + | | MeltingPt = | ||
| + | | MeltingPtC = 102 to 103 | ||
| + | | MeltingPt_ref = | ||
| + | | MeltingPt_notes = | ||
| + | | pKa = | ||
| + | | pKb = | ||
| + | | Solubility = 143 g/L | ||
| + | | SolubleOther = 237 g/L (15°C) in [[ethanol]]<hr>14 g/L (15°C) in [[diethyl ether]] | ||
| + | | Solvent = | ||
| + | | VaporPressure = | ||
| + | }} | ||
| + | | Section3 = {{Chembox Structure | ||
| + | | Coordination = | ||
| + | | CrystalStruct = | ||
| + | | MolShape = | ||
| + | }} | ||
| + | | Section4 = {{Chembox Thermochemistry | ||
| + | | DeltaGf = | ||
| + | | DeltaHc = | ||
| + | | DeltaHf = | ||
| + | | Entropy = | ||
| + | | HeatCapacity = | ||
| + | }} | ||
| + | | Section5 = {{Chembox Explosive | ||
| + | | ShockSens = | ||
| + | | FrictionSens = | ||
| + | | DetonationV = | ||
| + | | REFactor = | ||
| + | }} | ||
| + | | Section6 = {{Chembox Hazards | ||
| + | | AutoignitionPt = | ||
| + | | ExploLimits = | ||
| + | | ExternalMSDS = | ||
| + | | FlashPt = 166°C | ||
| + | | LD50 = | ||
| + | | LC50 = | ||
| + | | MainHazards = | ||
| + | | NFPA-F = | ||
| + | | NFPA-H = | ||
| + | | NFPA-R = | ||
| + | | NFPA-S = | ||
| + | }} | ||
| + | | Section7 = {{Chembox Related | ||
| + | | OtherAnions = | ||
| + | | OtherCations = | ||
| + | | OtherFunction = | ||
| + | | OtherFunction_label = | ||
| + | | OtherCompounds = [[:Category:Oxalates|Oxalates]] | ||
| + | }} | ||
| + | }} | ||
'''Oxalic acid''' is an organic compound with the chemical formula H<sub>2</sub>C<sub>2</sub>O<sub>4</sub>. Along with [[formic acid]], it is one of the most corrosive organic acids. | '''Oxalic acid''' is an organic compound with the chemical formula H<sub>2</sub>C<sub>2</sub>O<sub>4</sub>. Along with [[formic acid]], it is one of the most corrosive organic acids. | ||
| Line 31: | Line 143: | ||
==Handling== | ==Handling== | ||
===Safety=== | ===Safety=== | ||
| − | Oxalic acid is corrosive to human tissues, so proper protection must be worn. It is not | + | Oxalic acid is corrosive to human tissues, so proper protection must be worn. It is not volatile under normal conditions, so its vapors aren't usually a hazard. Oxalates are the notorious cause of kidney stones, so ingestion of both acid and salt should be avoided. |
===Storage=== | ===Storage=== | ||
| Line 50: | Line 162: | ||
[[Category:Carboxylic acids]] | [[Category:Carboxylic acids]] | ||
[[Category:Corrosive chemicals]] | [[Category:Corrosive chemicals]] | ||
| + | [[Category:Oxalates]] | ||
Revision as of 20:27, 4 August 2015

| |
| |
| Names | |
|---|---|
| IUPAC name
Oxalic acid
| |
| Systematic IUPAC name
Ethanedioic acid | |
| Properties | |
| H2C2O4 | |
| Molar mass | 90.035 (anhydrous) 126.066 (dihydrate) |
| Appearance | White crystals |
| Odor | Odorless |
| Melting point | 102 to 103 °C (216 to 217 °F; 375 to 376 K) |
| 143 g/L | |
| Solubility | 237 g/L (15°C) in ethanol 14 g/L (15°C) in diethyl ether |
| Hazards | |
| Flash point | 166°C |
| Related compounds | |
| Related compounds
|
Oxalates |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
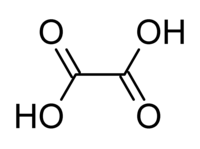
Oxalic acid is an organic compound with the chemical formula H2C2O4. Along with formic acid, it is one of the most corrosive organic acids.
Contents
Properties
Chemical
Oxalic acid is very strong for an organic acid. It will react with manganese dioxide to form manganese oxalate and carbon dioxide.
- MnO2 + 2 H2C2O4 → MnC2O4 + 2 CO2 + 2H2O
Physical
Oxalic acid is a colorless hygroscopic crystalline solid, with a snow-like aspect. It is soluble in water (143 g/L at 25˚C), more soluble in ethanol (240 g/L) but poorly soluble in ether (18 g/L). It has a weak smell and is irritating to skin. It has a melting point of 102 °C and sublimes between 149 - 160 °C.
Availability
Oxalic acid is sometimes available as wood bleach or as beehive disinfecting powder. It can also be bought online, such as from Amazon. "Bar Keepers Friend" is a very cheap multi-surface cleaner containing oxalic acid dihydrate, available at hardware stores.
Preparation
Oxalic acid can be made from the oxidation of sucrose, glucose, or ethylene glycol using nitric acid or air in the presence of vanadium pentoxide.
Below is a synthesis of oxalic acid found online, using nitric acid and sucrose:
10 g of sugar are added in a flat bottom flask and 50 ml of concentrated nitric acid and heat the flask in a water bath. The reaction will yield nitrogen oxide fumes, so it's best performed outside or in a fume hood. Stop the heating and remove the flask from the water bath. When the reaction subsides, add the hot solution into an evaporating basin. Wash out the flask with 10 ml of conc. nitric acid and evaporate the solution on the water bath until it has a volume of 10 ml. Add 20 ml of water to the solution and evaporate again to 10 ml. Cool the solution in a cooling bath to crystallize the oxalic acid. Dry the crystals.[1]
Projects
- Purifying MnO2 from old batteries
- Making formic acid by distillation of oxalic acid with glycerol
- Lanthanide purification
- Make pyrophoric iron
- Oxalate esters
Handling
Safety
Oxalic acid is corrosive to human tissues, so proper protection must be worn. It is not volatile under normal conditions, so its vapors aren't usually a hazard. Oxalates are the notorious cause of kidney stones, so ingestion of both acid and salt should be avoided.
Storage
Should be stored in closed bottles.
Disposal
Oxalic acid and oxalates can be destroyed with hydrogen peroxide.[2]
References
- ↑ http://www.scribd.com/doc/45869039/preparation-of-Oxalic-acid
- ↑ http://web.ornl.gov/info/reports/1981/3445605762877.pdf