Difference between revisions of "Urea"
Brain&Force (Talk | contribs) (Created page with "220x220px '''Urea''' or '''carbamide''', is an organic compound commonly used as a fertilizer. It has the chemical formula (NH<sub>2</sub>)<sub>2</sub>...") |
(→Properties) |
||
| Line 6: | Line 6: | ||
Thermal decomposition of urea produces ammonium isocyanate, who in turn yields isocyanuric acid. | Thermal decomposition of urea produces ammonium isocyanate, who in turn yields isocyanuric acid. | ||
:NH<sub>2</sub>CONH<sub>2</sub> → NH<sub>4</sub>NCO → HNCO + NH<sub>3</sub> | :NH<sub>2</sub>CONH<sub>2</sub> → NH<sub>4</sub>NCO → HNCO + NH<sub>3</sub> | ||
| + | |||
| + | Urea is a weak organic base. It forms salts with strong acids. | ||
===Physical=== | ===Physical=== | ||
Revision as of 16:05, 3 August 2015
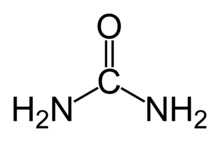
Urea or carbamide, is an organic compound commonly used as a fertilizer. It has the chemical formula (NH2)2CO. Urea was the first organic chemical produced from inorganic chemicals, a breakthrough in chemistry, which proved organic substances can be produced from inorganic substances, disproving the notion of vitalism.
Contents
Properties
Chemical
Thermal decomposition of urea produces ammonium isocyanate, who in turn yields isocyanuric acid.
- NH2CONH2 → NH4NCO → HNCO + NH3
Urea is a weak organic base. It forms salts with strong acids.
Physical
Urea is a white crystalline substance. Its melting point is between 133-135 °C. Urea is soluble in water (107.9 g/100 ml at 20 °C) and when dissolved, it is neutral on the pH scale. Urea is soluble in glycerol (500 g/L) and slightly less soluble in ethanol.
Availability
Urea is available as a fertilizer, either pure or mixed with other substances. It can be very cheaply purchased online, often at less than $5/kg.
ScienceCompany sells 500g of lab grade urea at 16.95 $.
Preparation
Urea was first synthesized by reacting silver cyanate with ammonium chloride.
- AgNCO + NH4Cl → (NH2)2CO + AgCl
Industrially it is produced by the thermal decomposition of ammonium carbamate.
It can also be produced from the reaction of phosgene and ammonia
- COCl2 + 4 NH3 → (NH2)2CO + 2 NH4Cl
Projects
- Urea nitrate synthesis
- Sodium azide synthesis
- Hydrazine sulfate(Hoffman Rearrangement)
Handling
Safety
Urea is non-toxic, though is may cause irritations to sensitive tissues. It may corrode metallic surfaces, even the more resistant forms of stainless steel.
Storage
Urea doesn't require any special storage, and can be safely stored in glass, metal, plastic or ceramic containers.
Disposal
As urea is a good fertilizer, it can be safely dumped in the soil, although it's recommended to avoid dumping it in water reservoirs to prevent the growth on unwanted algae.