
This renders the use of the anhydride rather academic, wouldn't you agree? At least on a prep scale, and what else are we talking about here?

 Ah! Those youthfull days of dabbling.
Ah! Those youthfull days of dabbling.Quote: Originally posted by Sauron  |
Quote: Originally posted by Sauron  |
| Quote: |
 enough to buy KI. My goal is only to beat the price of $80 for 50g of MeI. This is the price from the only place I could find it (that would sell it
to me). I think phosphoric acid can work pretty well here.
enough to buy KI. My goal is only to beat the price of $80 for 50g of MeI. This is the price from the only place I could find it (that would sell it
to me). I think phosphoric acid can work pretty well here. maybe publish some home chemistry in Nature or Science.
maybe publish some home chemistry in Nature or Science.Quote: Originally posted by Sauron  |
Quote: Originally posted by Sauron  |
 . What I just did is some testing on
a small test tube scale to get a feeling of what I can expect and how things behave. I always do that kind of tests before I use larger quantities.
. What I just did is some testing on
a small test tube scale to get a feeling of what I can expect and how things behave. I always do that kind of tests before I use larger quantities. 



Quote: Originally posted by Sauron  |
| Quote: |
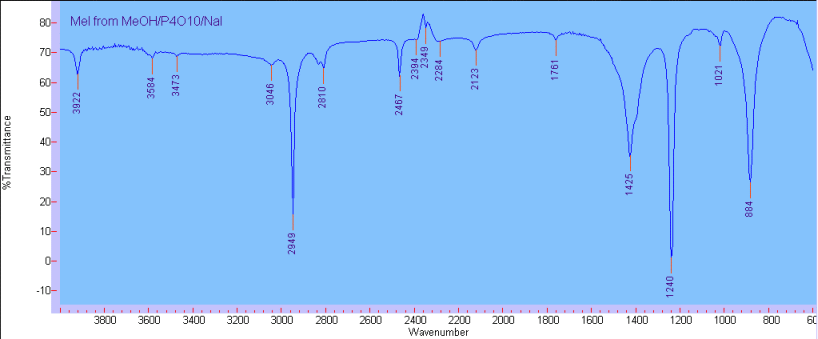


| Quote: |
 .
.  ).
).Quote: Originally posted by Sauron  |
Quote: Originally posted by PainKilla  |
Quote: Originally posted by Sauron  |
 ) then going by the methyl bromide may be more
"convenient". However if you also have equal stock of methyl tosylate (or tosyl chloride) then this would be the most satisfactory way to proceed. The
volatility/low bpt of MeBr is easily made negligable as I suggested above, supposing you have dry ice (which you can make from your tank of CO2).
) then going by the methyl bromide may be more
"convenient". However if you also have equal stock of methyl tosylate (or tosyl chloride) then this would be the most satisfactory way to proceed. The
volatility/low bpt of MeBr is easily made negligable as I suggested above, supposing you have dry ice (which you can make from your tank of CO2).Quote: Originally posted by Sauron  |