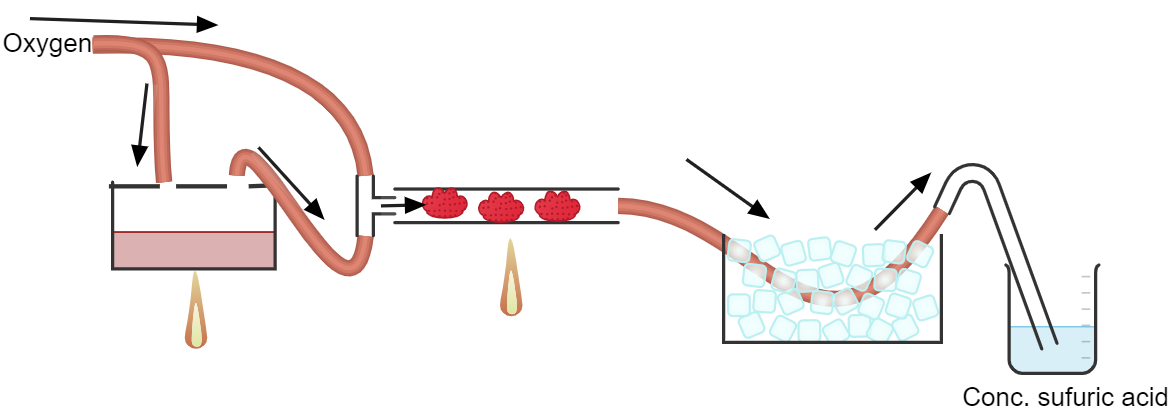
| I might give iron oxide a go. Using pure oxygen instead of atmospheric oxygen should increase the conversion rates significantly I think. I actually
have no idea how to can setup a catalyst bed of iron oxide. I can use a gas stove to heat it up but it will be hard to control the temperature. So,
how can I setup an iron oxide catalyst bed? |