| Pages:
1
..
8
9
10
11
12
..
25 |
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
Apples and oranges only make a good sangria. Patents are essentially
exclusive business licenses and an article of commerce that inherently
seeks to retain proprietary it's described process or proceedure .
Journals are written for the disclosure of discovery and do not withhold
confidential know how. The later is for fame , the former is for fortune.
.
|
|
|
Douchermann
Hazard to Others
  
Posts: 117
Registered: 11-10-2005
Location: Illinois, USA
Member Is Offline
Mood: No Mood
|
|
Sorry to bring a thread up from the dead, but would performing the reaction in something other than glass affect it in any way? The reason I'm asking
is because the biggest glass piece I have is a 1000ml flask, and with the foaming, that wont be sufficient. However, I have a 5 gallon (18 liter)
aluminum pot that I could heat quite easily. If the aluminum pot affects it negatively, then I guess a Teflon coated cast iron pot would be out of
the question too, correct?
It\'s better to be pissed off than to be pissed on.
|
|
|
YeOldeImpurities
Harmless

Posts: 8
Registered: 23-12-2007
Location: Europe
Member Is Offline
Mood: D'oh!
|
|
higher yields of hydrazine?
Compounds of manganese (sulfate, chloride of manganese, permanganates...) in hypochlorite/urea/hydroxide reaction work better than gelatine. They give
5-10% higher yields of hydrazine than mixtures with gelatine. --- this I remember from book "The Chemistry of Hydrazine" by Audrieth and Ogg.
In russian book Греков,Отрошко:
"Гидразинометрия" 1981 (Hydrazinometry by Grekov and
Otroshko) is a recipe for making hydrazine sulfate with MnSO4.5H2O instead of gelatine (0,3 grams of MnSO4.5H2O to 15 grams of urea, yield 23 grams of
hydrazine sulfate).
And also something else for increasing yields- in book
Брикун,Козловский,Никит&
#1080;на: "Гидразин и
гидроксиламин и их
применение в
аналитической химии" 1967 (Hydrazine and
hydroxylamine nad their application in analytical chemistry by Brikun, Kozlovskiy and Nikitina) is this: best compounds for protection of
hydrazine/water solutions against oxidizing by air are oxides of calcium, zinc, aluminium and magnesium but the far superior is sulfur in small
amounts. btw there is mentioned a method for producing hydrazine by dry destillation of urea/iron powder at 132-150ºC at atmospheric pressure.
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by Sauron
I have never seen a prep deleted from Org.Syn....
I don't question that in some text, somewhere, what you are saying may be happening. But I challenge you to show me an example from Org.Syn.
... |
Sorry, missed this reply back when it was done
Not that the actual earlier prep is deleted, but especially in the earlier collected series you would see mention made that XYZ was now commercially
available, and either stated or implied that DIY wasn't worth it.
OS and IS don't change their reprints, the data remains unchanged. However textbooks do drop entries, I've several cases of multiple editions of the
same book where some preparations were dropped and usually new ones added. And before OS was available online, textbooks were more likely to be found
as affordable used books.
There's also changes in reagents across time. In the organic world it was typical to see an old method be dropped for one using a more exotic reagent
but giving higher yields and/or more easily isolatable product. Over in the inorganic world an example in 'practical' books was in the preparation of
HBr; first the SO2 and H2S routes disappeared, leaving just the red phosphorus method, then the preparation was dropped entirely as HBr became an easy
to obtain reagent. But nowadays an amateur experimenter may not be able to purchase HBr, not unlikely for liability issues, not able to buy
phosphorus because of legal restrictions, and thus be looking for those old alternatives that don't get mentioned in modern books.
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by Douchermann
Sorry to bring a thread up from the dead, but would performing the reaction in something other than glass affect it in any way? The reason I'm asking
is because the biggest glass piece I have is a 1000ml flask, and with the foaming, that wont be sufficient. However, I have a 5 gallon (18 liter)
aluminum pot that I could heat quite easily. If the aluminum pot affects it negatively, then I guess a Teflon coated cast iron pot would be out of
the question too, correct? |
The metal pot might react with the alkali, the non-stick coating if new should be OK.
|
|
|
len1
National Hazard
   
Posts: 595
Registered: 1-3-2007
Member Is Offline
Mood: NZ 1 (goal) - Italy 1 (dive)
|
|
| Quote: | | There are often good reasons why, even for perfectly good ideas, some vital details are left out of patent descriptions. Most notably it makes it more
difficult for someone to pirate the idea. |
Maybe, but this strikes me as a very dangerous thing to do. If someone subsequently reinvents this 'omission' and makes the procedure work, the
patent holder has a good chance of getting no royalties.
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
About - higher yields of hydrazine ?
Welcome YeOldeImpurities
Very intriguing references , particularly the last about distillation , although
that may pose an explosion risk similar to distillation of H2O2. Iron normally
interferes with hypochlorite processes. By the way, this can't be right,
it's not stoichiometric -
0,3 grams of MnSO4.5H2O to 15 grams of urea, yield 23 grams of hydrazine sulfate
Please post more details if you can, transactions here are in english, few
can read cyrillic. As you see the postings tend to deviate into side issues
unrelated to the main thread. Don't be put off by this, consider starting
another thread of your own.
.
|
|
|
YeOldeImpurities
Harmless

Posts: 8
Registered: 23-12-2007
Location: Europe
Member Is Offline
Mood: D'oh!
|
|
Hi Franklyn, here is an approximate translation from that book: Add concentrated solution of 0.3g of MnSO4.5H2O to 60ml of cooled NaOH solution of
density 1.37-1.38. At 0 degrees of celsius add 15g of urea, then cool the solution to -7 degrees. Then gradually add 125ml of NaClO solution cooled to
-3 degrees. Do not allow to rise the temperature of the solution over +10 degrees. When NaClO was added, let it stand for 10 minutes. Then heat the
solution gradually, 2 degrees per 1 minute in water bath to +63-65 degrees. Then allow to cool to room temperature and add the solution gradually to
stirred 140ml of 50% H2SO4. Do not allow to rise the temperature over 40-50 degrees. Then cool to +22-25 degrees and filter out the crystals of
hydrazine sulfate and wash them three times with 10 ml of water and dry. Yield 23 grams (70% of the theoretical).
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
NH2NH2 from KCN, KHSO4, KNO2
Laboratory Methods of Inorganic Chemistry
Heinrich Biltz, Wilhelm Biltz
http://books.google.com/books?id=1gkNAAAAYAAJ&pg=PA215&a...
From prep 122 on page 158

|
|
|
Mason_Grand_ANNdrews
Hazard to Self
 
Posts: 63
Registered: 19-1-2006
Location: New Berlin City !!
Member Is Offline
Mood: crabby
|
|
Excuse to write the text into this, it is my second time to write down that. I`ve sitting down, hove some nice ideas and my editor was killed by what
you have now 5 minutes to hold. I thkink - it was ""never mind""
I hope it is somewhat for the "balconery" chemistry to get hydrazine.
Prepare two saturated solutions of 4 mol ammonium chloride and 0.5 mol of sodium chloride. Combine the solutions in a beaker in an salt ice bad to
minimum 0 degrees centigrade (ammonium) nitrate ice bad is better when you have) that it not will react anytime. A third solution of 6 mol of 25 %
ammonium hydroxide is chilled to 0 degrees centigrade and combined with the first mixture in an distiller and put on an distiller by stirring it with
an teflon rod.
Step one:
Heat the mix over many hours to the lowest bp of all the liquids
(water 101 degrees centigrade) and put the condenser in an ice bad by connecting the equipment to an disstillation equpment and an simple vacuum pump
or an electircal when you have. The distilled liquid is collected in an chilled beaker of ammonium nitrate
ice and connectect to a new eqiupment.
Step 2:
Hold the distillate under cold conditons below 0 degrees centigrade and connect it to an new disstillation equipment with two mol of 25 % HCl in the
condenser. The condenser is chilled with an sligh ammonium nitrate ice bad and the yieded liquid is transferd into a new beaker by collecting this
to a new disstillation equipment.
Step 3:
Prepare an ""condenser"" with 2,5 mol of 25 % HCl and connect this to the chilled distiller by step two and over a few hours rise the temperature of
the distillate to over 190 degrees centirade and remove the most of the water if you can. The stuff is dryed by placed it to an easy filter paper and
hang it over an mix of magnesium sulfate powder and the water is removed over al lot of days.
Soo, i hope this is an real alternative to some hard to get stuff and what you can get from nothing. I`ve to much trunken but i hope it helps.
Money is fare away besides - but i`m an illusian.
By the way, the hydrazinedihydrochloride what the synth. calculated by me is dryed over concentrated sodium hydoxyde or something stuff wath is
hycroscopic. I mean it works when the slick is placed into an filter and dryed over concentrated H2SO4 by connecting a vacuum pump to the
flask !
I hope it sometime easy or correct, some hints will help.
It`s very pity that i`ve no lab more that a few years, it more
than an feeling. Maybe someone can this test if it work - i hope.

[Edited on 4-3-2008 by Mason_Grand_ANNdrews]
Every love is a little inspiration - every day is
a new live !
|
|
|
Formatik
National Hazard
   
Posts: 927
Registered: 25-3-2008
Member Is Offline
Mood: equilibrium
|
|
| Quote: | Originally posted by YeOldeImpurities
Compounds of manganese (sulfate, chloride of manganese, permanganates...) in hypochlorite/urea/hydroxide reaction work better than gelatine. They give
5-10% higher yields of hydrazine than mixtures with gelatine. --- this I remember from book "The Chemistry of Hydrazine" by Audrieth and Ogg.
In russian book Греков,Отрошко:
"Гидразинометрия" 1981 (Hydrazinometry by Grekov and
Otroshko) is a recipe for making hydrazine sulfate with MnSO4.5H2O instead of gelatine (0,3 grams of MnSO4.5H2O to 15 grams of urea, yield 23 grams of
hydrazine sulfate).
And also something else for increasing yields- in book
Брикун,Козловский,Никит&
#1080;на: "Гидразин и
гидроксиламин и их
применение в
аналитической химии" 1967 (Hydrazine and
hydroxylamine nad their application in analytical chemistry by Brikun, Kozlovskiy and Nikitina) is this: best compounds for protection of
hydrazine/water solutions against oxidizing by air are oxides of calcium, zinc, aluminium and magnesium but the far superior is sulfur in small
amounts. btw there is mentioned a method for producing hydrazine by dry destillation of urea/iron powder at 132-150ºC at atmospheric pressure.
|
What yield is that of the theory? Raschig in German patent #198307 says hypochlorite solution and ammonia with lime can get as much as 80 percent of
the theory, and he himself gets 60 to 70 percent in an example.
|
|
|
Formatik
National Hazard
   
Posts: 927
Registered: 25-3-2008
Member Is Offline
Mood: equilibrium
|
|
| Quote: | Originally posted by Rosco Bodine
GB1073292 Hydrazinium Cyanurate
This is another means of precipitating a slightly soluble hydrazine salt which may be freebased by NaOH . |
There are also other hydrazonium salts which might be worth trying. Although the oxalate is soluble, hydrazonium hydrooxalate N2H5HC2O4 is said to
result from estimated amounts of oxalic acid and N2H5OH, and these crystals are barely soluble in cold water: 2.02 g per 100 g H2O at 22.5 deg.
Hydrazonium subhydrophosphate N2H5H3P2O6 made by neutralizing hypophosphoric acid with N2H5OH against methyl orange and addition of the same amounts
of hypophosphoric acid. This salt is even less soluble, 1.5 g per 100 g H2O at room temperature. Compared with the hydrosulfate, the solubilities are
for 100 g H2O: 2.794 g at 20 deg, 3.302 at 25 deg., 6.538 at 50 deg., 12.580 at 80 deg., etc. which solubility is also decreased by acids.
Another idea is hydrazonium pyrosulfite (N2H5)2S2O5 which is made by leading so much SO2 into N2H5OH soln. until it turns yellow, this evolves
considerable heat. Allowing this to stand in a vaccum or in an SO2 atmosphere, or by precipitating with alcohol causes it to crystallize. The salt
also oxidizes easily to sulfate. I bubbled SO2 into a small amount of a weeks old reacted liquor solution of urea to test this, the solution got warm
from bubbling and eventually was very barely yellow, and let the solution stand in a closed container for a few hours. At the bottom there was a small
amount of glittering, glancing white crystals. Best to try on larger scale, but I would say using the hydrosulfate is more advantageous.
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
Syntheses of some N-substituted hydrazines by the anhydrous chloramine process
S R Jain and D Chappella
Proceedings, Indian Academy of Science
Chemical Science Journal , Vol 95 , pp 381 - 389
October 1985
Attachment: N-substituted hydrazines by anhydrous chloramine.pdf (1.9MB)
This file has been downloaded 1435 times
|
|
|
DeAdFX
Hazard to Others
  
Posts: 339
Registered: 1-7-2005
Location: Brothel
Member Is Offline
Mood: @%&$ing hardcore baby
|
|
hmmm here is a patent discussing the usage of Ca(OCl)2 as a chlorine source as opposed to NaOCl. I skimmed over the patent a little bit but it certainly does
seem interesting. Is their any real benefit in using the ketazine process over the Olin Raschig process?
[Edited on 9-8-2008 by DeAdFX]
|
|
|
mbrown3391
Hazard to Others
  
Posts: 133
Registered: 2-9-2006
Member Is Offline
Mood: No Mood
|
|
Hydrazine from Acetone, Ammonia and Bleach
1. 2 CH3-C=O-CH3 + 2 NH3 + NaOCl --> CH3-CH3-C=N-N=C-CH3-CH3 + NaCl + 3H2O
2. CH3-CH3-C=N-N=C-CH3-CH3 + 2 H2O --> H2N-NH2 + 2 CH3-C=O-CH3
Has anyone tried this method of preparing Hydrazine?
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
Post your referencess for this putative reaction. At present it appears at best incomplete and unlikely.
Sic gorgeamus a los subjectatus nunc.
|
|
|
chemoleo
Biochemicus Energeticus
    
Posts: 3005
Registered: 23-7-2003
Location: England Germany
Member Is Offline
Mood: crystalline
|
|
He's referring to the ketazine process, the industrial method of preparing hydrazine.
http://en.wikipedia.org/wiki/Ketazine_process
I seem to remember looking into this, does it perhaps apply high pressure? Somehow I must have concluded at the time that it is not easily possible
for the amateur. I don't have my references here, so maybe someone else can help on the details.
Never Stop to Begin, and Never Begin to Stop...
Tolerance is good. But not with the intolerant! (Wilhelm Busch)
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
Pretty much what I meant by "incomplete and unlikely"
I have seen acetone cyanohydrin reacted with ammonia, then coupled to the substituted hydrazine, then oxidized to AIBN, the radical initiatyor. But,
using that approach then expecting to end up reversing it, keeping the N-N bond while cleaving two C-N bonds?
Sounds like a job for some supercatalyst and harsh conditions.
Of course he could ask Rosco about urea -> hydrazine.
Sic gorgeamus a los subjectatus nunc.
|
|
|
ScienceSquirrel
International Hazard
    
Posts: 1863
Registered: 18-6-2008
Location: Brittany
Member Is Offline
Mood: Dogs are pets but cats are little furry humans with four feet and self determination! 
|
|
It is definitely a practical approach on an industrial scale.
http://www.atsdr.cdc.gov/toxprofiles/tp100-c4.pdf
However if one is making small amounts of hydrazine in the lab then the classic approach is quite adequate and there are good recipes that if you
follow them faithfully will produce a good yield.
|
|
|
kmno4
International Hazard
    
Posts: 1495
Registered: 1-6-2005
Location: Silly, stupid country
Member Is Offline
Mood: No Mood
|
|
Oxidation NH3 with NaOCl is standar procedure for making N2H4.
Reaction with acetone has also application for making pure hydrazine salts. But for hydrolysis 30% H2SO4 is using. All these you can find (in
russian) in work of Kariakin and Angielov.
Besides, there is HYDRAZINE thread.
|
|
|
tapira1
Hazard to Others
  
Posts: 168
Registered: 9-10-2006
Location: Here!!!
Member Is Offline
Mood: 
|
|
NaClO + NH3
This works beautiful. The product may be crystallized as the sulfate
|
|
|
kilowatt
Hazard to Others
  
Posts: 322
Registered: 11-10-2007
Location: Montana
Member Is Offline
Mood: nitric
|
|
I did some poking around tonight, and it occurred to me that you could likely crystallize anhydrous hydrazine from any aqueous form significantly
exceeding 27% molar (40% w/w) at low temperature. I don't have a full phase diagram, but the eutectic is 27% molar N2H4 and freezes at 193°K. I
believe hydrazine hydrate (64% N2H4) begins to freeze at something like -50°C to -60°C. Thus, with dry ice it should be possible to crystallize an
amount of anhydrous hydrazine from hydrazine hydrate, leaving a liquid depleted to just over 40% N2H4. The diluted liquid could then be re-distilled.
Probably not the most efficient method, but it is interesting anyhow, and perhaps quite suitable for the lab.
The mind cannot decide the truth; it can only find the truth.
|
|
|
chloric1
International Hazard
    
Posts: 1136
Registered: 8-10-2003
Location: GroupVII of the periodic table
Member Is Offline
Mood: Stoichiometrically Balanced
|
|
Patent literature
Well, I read some patents that talk about the ketazine reaction. Although acetone would suffice, the product is water soluble and would be difficult
to separate under normal conditions. The patents I am thinking of uses MEK instead because mekazine is insoluble. This can be hydrolysized by
heating with strong acid at atmospheric pressure. A reflux setup here would probably be best here. The ketazine can be hydrolysized with plain
water. IIRC, it was at 250 C and 10 atm. This sounds bad but a heavy walled stainless tube with a bleed off valve might work. I seem to remmeber
they even described the pressure reactor. This would be dangerous but set up your workspace like an explosive bunker and you shoudl be OK.
Just remeber your cheap plastic soda bottle is good to like 8 or 9 atms!!
Fellow molecular manipulator
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
@ kilowatt
Interesting idea , but I think if you resort to temperature extremes
there are other methods perhaps better that become available also.
This reference contains data of interest , excerpts are seen below
An Assessment of Hydrazine, Hydrazine Hydrate and
Liquid Ammonia as fuels for Rocket Propulsion
K. A . Cooper & L. A. Wiseman - 1949
http://www.dtic.mil/cgi-bin/GetTRDoc?AD=ADA474005&Locati...
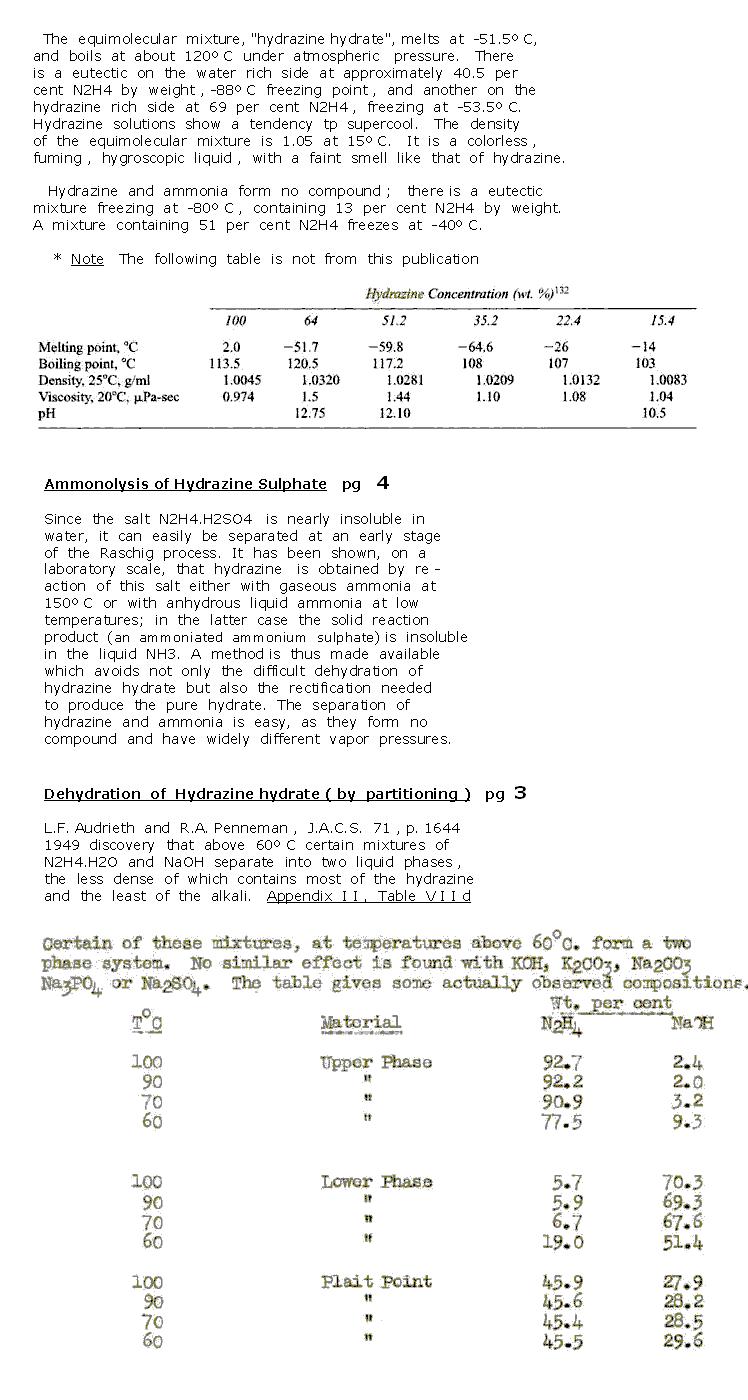
|
|
|
User
Hazard to Others
  
Posts: 339
Registered: 7-11-2008
Location: Earth
Member Is Offline
Mood: Passionate
|
|
Destroying hydrazine
Iam planning to throw myself on the hydrazine synthesis (Urea+hypo).
Can anyone tell me how it could destroy hydrazine(hydrate/sulfate) in a worst case scenario.
Grtz user.
|
|
|
| Pages:
1
..
8
9
10
11
12
..
25 |