| Pages:
1
..
17
18
19 |
oldtimer
Harmless

Posts: 12
Registered: 18-8-2008
Member Is Offline
Mood: No Mood
|
|
Where I live, 1-pentanol and "octyl alcohol" are easily available. I am however not sure, if the latter is 1-octanol or some branched isomer.
1-butanol I have in the lab.
I will try to prepare some 2-methyl-2pentanol, 2-methyl-2hexanol and 3-methyl-3-heptanol.
As to quantities: If I remember blogfasts posts correctly, he used about 5 .. 6 grams of alcohol for each of his potassium runs. 25 g should therefore
be a quantity that allows a few of these experiments.
|
|
|
Alice
Hazard to Others
  
Posts: 111
Registered: 11-5-2015
Member Is Offline
Mood: No Mood
|
|
In the meantime I had two new ideas about obtaining long chain tertiary alcohols.
My first idea was to alkylate raspberry ketone and perform a Grignard afterwards. The advtantage is to deal with solid products, thus easy
purification compared to high boiling and badly crystallyzing liquids.
Second, cyclization of citronellol with an acid in order to obtain either the alkene with further addition of water or dihydro terpineol directly. A
problem is to obtain citronellol. An alterternative synthesis is starting from citronellal, which needs to be reduced to citronellol first. A general
problem is to identfy reaction products. The advantage is to avoid reducing an alkene compared to the previously discussed synthesis of terpineol
followed by precious metal reduction to dihydro terpineol.
I think I'll try the raspberry ketone based synthesis first as I expect less pitfalls and the same iodoalkane can be used for both steps. Nevertheless
this may take a while...
[Edited on 17-4-2017 by Alice]
|
|
|
soniccd123
Harmless

Posts: 10
Registered: 20-1-2014
Location: Brazil
Member Is Offline
Mood: No Mood
|
|
Yesterday I was reading Semimicro and Macro Organic Chemistry by Nicholas Cheronis looking for sources on alcohol oxidation to carboxylic acids and
something caugh my atention: "The use of permanganate with alcohols
which contain tertiary hydrogen (branched chains), as for example,
isobutyl alcohol, (CH3)2CHCH2OH, involves the danger of oxidation
in other parts of the molecules." (page 199). I looked into more information on the internet about this and, while little was found, two papers (on
the end of the message) talk about the oxidation of tertiary hydrogens to hydroxyl groups.
Wouldn't be possible to oxidate the tertiary hydrogen on Isovaleric acid to 3-metil-3-hydroxybutanoic acid and then decarboxylate this to T-butanol?
Sketch of the reaction:
[bad img]http://imgur.com/a/HJL8i[/bad img]
Papers:
http://pubs.acs.org/doi/abs/10.1021/ja00705a642?journalCode=...
http://pubs.acs.org/doi/abs/10.1021/ja01501a033?journalCode=...
|
|
|
12thealchemist
Hazard to Others
  
Posts: 181
Registered: 1-1-2014
Location: The Isle of Albion
Member Is Offline
Mood: Rare and Earthy
|
|
I'm aware this thread hasn't been active for nearly a year, but I had an idea that may be worth considering before discarding as too much effort.
I was reading the potassium thread out of interest (the train of thought initiated by NurdRage's recent video on making sodium via that process) and
was thinking about alternative tertiary alcohols. The proportion appears to be ~5 mol%, working from woelen's numbers on http://woelen.homescience.net/science/chem/exps/synthesis_K/...
On the basis of this, I started thinking about how one could avoid any side reactions, and possibly have a "perfect" catalyst. Tertiary alcohols are
used so that α-elimination products cannot occur; ethanol forming acetaldehyde, isopropanol forming acetone. These then polymerise, and form organic
gunk.
However, all these thus-far proposed tertiary alcohols do have β-hydrogens, and so could undergo elimination to form alkenes, thus deactivating the
catalyst. One could use tertiary alcohols that do not have β-hydrogens, but one then ends up with things like tri(t-butyl)methanol or
1,1,2,3,3-pentamethylcyclohexan-2-ol, which are difficult and complicated to synthesise (and very expensive) for obvious reasons. What if one used a
bridgehead alcohol, for example bicyclo[2.2.2]octan-1-ol? Elimination products cannot form to bridgeheads due the inherently strained nature of the
product. Similarly, nucleophilic substitution reactions cannot occur.
After a bit of thought, I came up with this synthesis. I figured it would be feasible for the determined amateur.
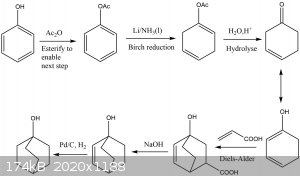
From a bit of googling, it appears that the Birch reduction cannot be carried out on phenol itself, unfortunately, hence the need to protect the OH. I
wonder if it is possible to combine the hydrolysis, Diels-Alder, and decarboxylation into a one-pot reaction, thereby speeding the process up
considerably. I am aware that the last step, the reduction, uses palladium on carbon, an expensive reagent, but I've seen it mentioned as being used
by other SM members, hence above claim of feasibility.
|
|
|
draculic acid69
International Hazard
    
Posts: 1371
Registered: 2-8-2018
Member Is Offline
|
|
NurdRage was talking about long chain acids for use in making an ester and couldn't seem to think of an easily obtainable source.
To quote NurdRage:
In a grignard, the alcohol side of the ester is lost.
As carbon sources themselves we'd need a long-chain carboxylic acid, i don't know of any other than acetic acid.
Stearic acid is cheap and easy to get on eBay.biodiesel is a long chain ester.
Both of these could be used in a grignard to get a long chain tertiary alcohol
couldn't they?
|
|
|
CuReUS
National Hazard
   
Posts: 928
Registered: 9-9-2014
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by 12thealchemist  | | What if one used a bridgehead alcohol, for example bicyclo[2.2.2]octan-1-ol? Elimination products cannot form to bridgeheads due the inherently
strained nature of the product. Similarly, nucleophilic substitution reactions cannot occur. |
That's a
brilliant idea  | Quote: | | I wonder if it is possible to combine the hydrolysis, Diels-Alder, and decarboxylation into a one-pot reaction, thereby speeding the process up
considerably. |
I don't think that's a good idea,since the water used in the hydrolysis would undergo a
michael addition to acrylic acid. | Quote: | | I am aware that the last step, the reduction, uses palladium on carbon, an expensive reagent, |
If you protect
the phenol as a benzyl ether,then Pd/C can be used to simultaneously reduce and deprotect in the last step.That would eliminate the need for the
hydrolysis step and make a one pot diels-alder/decarboxylation possible 
But your idea has given me another idea.Why not just buy amantadine and diazotize/hydrolyse it to alcohol ? Amantadine is sold as flu/parkinson med.
|
|
|
draculic acid69
International Hazard
    
Posts: 1371
Registered: 2-8-2018
Member Is Offline
|
|
Amantadine is prescription only.at 100 mg a pillow and a month supply of 30 tablets it is in no way feasible to use it for this purpose.the whole
turpineol route after19 pages yielded nothing.if I was going down this path I'd grignard some ethyl stearate made from easy to get metho and stearic
acid.sounds like a great starting point.
|
|
|
12thealchemist
Hazard to Others
  
Posts: 181
Registered: 1-1-2014
Location: The Isle of Albion
Member Is Offline
Mood: Rare and Earthy
|
|
Since this post, I gave the idea more thought, and thought "why bother with a mono-alcohol, make the molecule symmetrical, and make it a diol?" Then,
you should be able to use half the molar quantity from before, and have all the benefits outlined in the post above. Hence this proposal for
bicyclo[2.2.2]octan-1,4-diol :
The first couple of steps is given in an OrgSyn procedure,
Org. Synth. 1965, 45, 25
DOI: 10.15227/orgsyn.045.0025
http://www.orgsyn.org/demo.aspx?prep=CV5P0288
And the borate ester enolate protection is based on a short lecture course at university on Main Group elements in organic synthesis.
![Bicyclo[2.2.2]octane-1,4-diol.png - 205kB](http://www.sciencemadness.org/talk/files.php?pid=532232&aid=70205)
[Edited on 29-8-2018 by 12thealchemist]
|
|
|
CuReUS
National Hazard
   
Posts: 928
Registered: 9-9-2014
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by 12thealchemist  | | Since this post, I gave the idea more thought, and thought "why bother with a mono-alcohol, make the molecule symmetrical, and make it a diol?" Then,
you should be able to use half the molar quantity from before, and have all the benefits outlined in the post above. |
Even I suggested a diol idea in the previous page -http://www.sciencemadness.org/talk/viewthread.php?tid=15171&... | Quote: | | Hence this proposal for bicyclo[2.2.2]octan-1,4-diol |
isn't there a chance of polymerisation,having the OH's
at two poles ?
|
|
|
Vinylogous
Harmless

Posts: 17
Registered: 19-10-2011
Location: 'Murcah
Member Is Offline
Mood: Vilsmeier-Haacking
|
|
This stuff, Windflame PureFire Lamp oil, Windflame PureFire Lamp Oil, is pretty pure methyl coconoate, which is a distribution of C10-C18, with the majority being C12 (methyl laurate). Grignard on this with your bromoalkane of choice to get your tert alcohol
with a long linear tail and two symmetric "arms". Just my $0.02.
At some point in the next few months I would like to attempt making this HO(Et)subCC12 (from methyl laurate et al with
bromoethane), HOMeEtCC16 (from MEK and cetyl bromide), and maybe a few more of that ilk, and try them out for sodium.
HO(iAm)3 from isoamylbromide and isoamyl acetate might be fun too.
|
|
|
UC235
National Hazard
   
Posts: 565
Registered: 28-12-2014
Member Is Offline
Mood: No Mood
|
|
Walmart sells "coconut cooking oil" which advertises that it doesn't solidify. You can also buy a more pure material online called MCT Oil or
Capric/Caprylic Triglyceride (from supplement supply or cosmetic supply places). The former is what happens when you partially freeze coconut oil and
filter off the solids which removes much of the lauric and heavier containing triglycerides. What's left is highly enriched in hexanoic, octanoic,
decanoic, and oleic acids with a healthy amount of lauric left over. This material is easy to make into biodiesel (NaOH/KOH catalyzed
transesterification with a large excess of methanol) and the result is readily fractionated to give substantial amounts of methyl octanoate and methyl
decanoate.
MCT Oil is what happens when you distill the free fatty acids from coconut oil or palm kernel oil and re-esterify them with glycerol. It's essentially
mixed octanoic/decanoic triglyceride with a little hexanoic thrown in. The yields from processing this in the same manner as above will be even
higher.
You can also just buy isopropyl myristate as a cosmetic supply.
|
|
|
Vinylogous
Harmless

Posts: 17
Registered: 19-10-2011
Location: 'Murcah
Member Is Offline
Mood: Vilsmeier-Haacking
|
|
Triglycerides are just esters, so couldn't one just react a Grignard with the MCT oil directly? Having a hard time finding literature for this one,
but this source New Trends in Lipid and Lipoprotein Analyses, is promising.
[url=https://books.google.com/books?id=-Twl5OI0VgQC&pg=PA242&lpg=PA242&dq=grignard+on+"triglyceride"+ester&source=bl&ots=4RCrkAWM6
3&sig=eLph-BIwAmaCBvoQXhTsQqgVCwA&hl=en&sa=X&ved=2ahUKEwj9tv_2u8LdAhWJTt8KHaeEC9sQ6AEwDnoECAIQAQ#v=onepage&f=false]link[/url]
edit: the board does not like this link, you will have to manually paste it I think. "Contribution of Grignard Reagents in the Analysis of Short-Chain
Fatty Acids", p 242
| Quote: |
Application of Grignard Reagents to Oils and Fats Analysis
Two applications successfully used Grignard reagents in oils and fats analysis. One was analyzing the fatty acid regiodistribution in triglycerides.
The nonselectivity regarding fatty acid chains and nonregioselectivity of Grignard reagents was used to start operations through partial deacylation.
The brief, controlled degredation resulted in a mixture of diglycerides. The second involved analyzing wax constitutents, particularly jojoba.
Ethylmagnesium bromide rapidly attacked the ester bonds, and in a single step completed transformed jojoba wax rapidly and completely into a mixture
of tertiary alcohols that corresponded to the fatty acids and native primary alcohols. The mixture of alcohols was analyzed directly in gas
chromatography with a single injection.
|
In theory, with enough Grignard, the triglyceride should be ripped to shreds, affording the tris-MgBr adduct of glycerol and the deprotonated tert
alcohol- MgBr+, upon quench giving the tert alcohol and glycerol. I think the key phrasing is controlled
degredation, which leads me to believe they tried to carefully strip off one fatty acid.
This source, Studies on the positional specificity of lipase from Mucor miehei during interesterification reactions of cod liver oil with n-3 polyunsaturated
fatty acid... by Haraldsson and Almarsson, 1991, suggests that even with 6 moles of MeMgBr to 1.1 mol triglyceride, diglyceride was obtained.
Again, that's not an excess of Grignard, so I think they are trying to do something to partially degrade the fats for the purpose of analysis.
[Edited on 17-9-2018 by Vinylogous]
[Edited on 17-9-2018 by Vinylogous]
|
|
|
draculic acid69
International Hazard
    
Posts: 1371
Registered: 2-8-2018
Member Is Offline
|
|
NurdRage just posted a video using 4OHterpineol from purified tea tree oil for the sodium metal rxn on YouTube with good results.seems like this is
the way to go.
[Edited on 16-10-2018 by draculic acid69]
|
|
|
draculic acid69
International Hazard
    
Posts: 1371
Registered: 2-8-2018
Member Is Offline
|
|
NurdRage last video shows long chain secondary alcohol s work in the NaOH/mg rxn.they seem to have a lower yield but they work.menthol crystals on
eBay or from mint flavorings for cooking or vaping are otc and ready to go.
[Edited on 17-11-2018 by draculic acid69]
|
|
|
draculic acid69
International Hazard
    
Posts: 1371
Registered: 2-8-2018
Member Is Offline
|
|
In the spirit of this post where nothing seems to work out can I just say can no-one suggest extracting the menthol from cigarettes to use in this
rxn.just buy it it's otc enough.
|
|
|
draculic acid69
International Hazard
    
Posts: 1371
Registered: 2-8-2018
Member Is Offline
|
|
Menthol can give 90% yield of sodium.no need for tert alcohols.
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2781
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
I had wondered if terpenes would work here. Menthol is a secondary alcohol but it's very hindered. Terpineol is a tertiary alcohol which IIRC can be
distilled from pine oil.
[Edited on 16-12-2018 by clearly_not_atara]
|
|
|
Texium
Administrator
       
Posts: 4566
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
Wouldn't a fully saturated tertiary alcohol such as 2-methyl-2-decanol still be a cleaner and more effective catalyst, in theory?
|
|
|
draculic acid69
International Hazard
    
Posts: 1371
Registered: 2-8-2018
Member Is Offline
|
|
Watch Nurdrages latest vid on YT it will explain everything.
|
|
|
draculic acid69
International Hazard
    
Posts: 1371
Registered: 2-8-2018
Member Is Offline
|
|
Steric hindrance actually helps the rxn the longer chain part doesn't participate as much as previously thought
Borneol gets the rxn done in 10 hrs compared to 20-30 for menthol and long chain tert alcohols. But menthol is by far cheaper and more OTC.
[Edited on 21-12-2018 by draculic acid69]
|
|
|
nora_summers
Harmless

Posts: 34
Registered: 11-11-2010
Member Is Offline
Mood: I am figment of your imagination.
|
|
This sticky is 9 years old and has pretty much been rendered obsolete in light of recent work. Do we still need to keep this stickied?
|
|
|
j_sum1
Administrator
       
Posts: 6307
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
Just because a solution has been found for the primary application does not mean there is no benefit to the contents of this thread. And I know that
I intend to give it a good read before attempting Na synthesis using menthol catalyst. Rb and Cs are still to be investigated. Li too perhaps. So
there is no good reason to unsticky this. It is not like there is any major inconvenience to having it near the top of the list.
|
|
|
nora_summers
Harmless

Posts: 34
Registered: 11-11-2010
Member Is Offline
Mood: I am figment of your imagination.
|
|
On the other side of the coin, there is no good reason to sticky this thread in particular. There are hundreds of other threads of interesting and
more pertinent chemistry. Why is this one special enough to be stickied? Let's sticky some other very interesting threads like the Ketene lamp or OTC
safe methylating agents.
|
|
|
12thealchemist
Hazard to Others
  
Posts: 181
Registered: 1-1-2014
Location: The Isle of Albion
Member Is Offline
Mood: Rare and Earthy
|
|
Rubidium has recently been synthesised by Pok over on the potassium thread and the "new" German chemistry forum
|
|
|
Cou
National Hazard
   
Posts: 958
Registered: 16-5-2013
Member Is Offline
Mood: Mad Scientist
|
|
I've been wondering if the Steglich esterification would work for tertiary alcohols such 4-propylheptan-4-ol (made from a butyrate ester and 2
equivalents of propylmagnesium bromide).
https://www.organic-chemistry.org/namedreactions/steglich-es...
It works for making esters of tert-butyl alcohol, but the original publication doesn't give examples of yields for even more bulky alcohols.
It would be interesting to find out what the esters of these long-chain tertiary alcohols smell like. Eventually I'll find out for myself if the
steglich esterification works, or if I have to use acid chlorides.
Simple Method for the Esterification of Carboxylic Acids
B. Neises, W. Steglich, Angew. Chem. Int. Ed., 1978, 17, 522-524.
[Edited on 7-3-2020 by Cou]
|
|
|
Texium
|
Thread Untopped
29-11-2023 at 09:12 |
| Pages:
1
..
17
18
19 |