| Quote: |
| Originally posted by Barium Steve, which substrate have you tried nickel boride on? |
Not allowed to say
 I'd much rather use Zn/H+ any day...
I'd much rather use Zn/H+ any day... 

















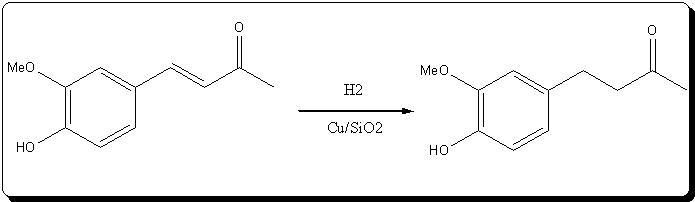










| Quote: |
 ) or you're using a DEhydrogenation catalyst for hydrogenation?
) or you're using a DEhydrogenation catalyst for hydrogenation?| Quote: |
| Quote: |
 I'd much rather use Zn/H+ any day...
I'd much rather use Zn/H+ any day...| Quote: |
| Quote: |

| Quote: |
| Quote: |