
Ritter - 28-7-2008 at 08:09
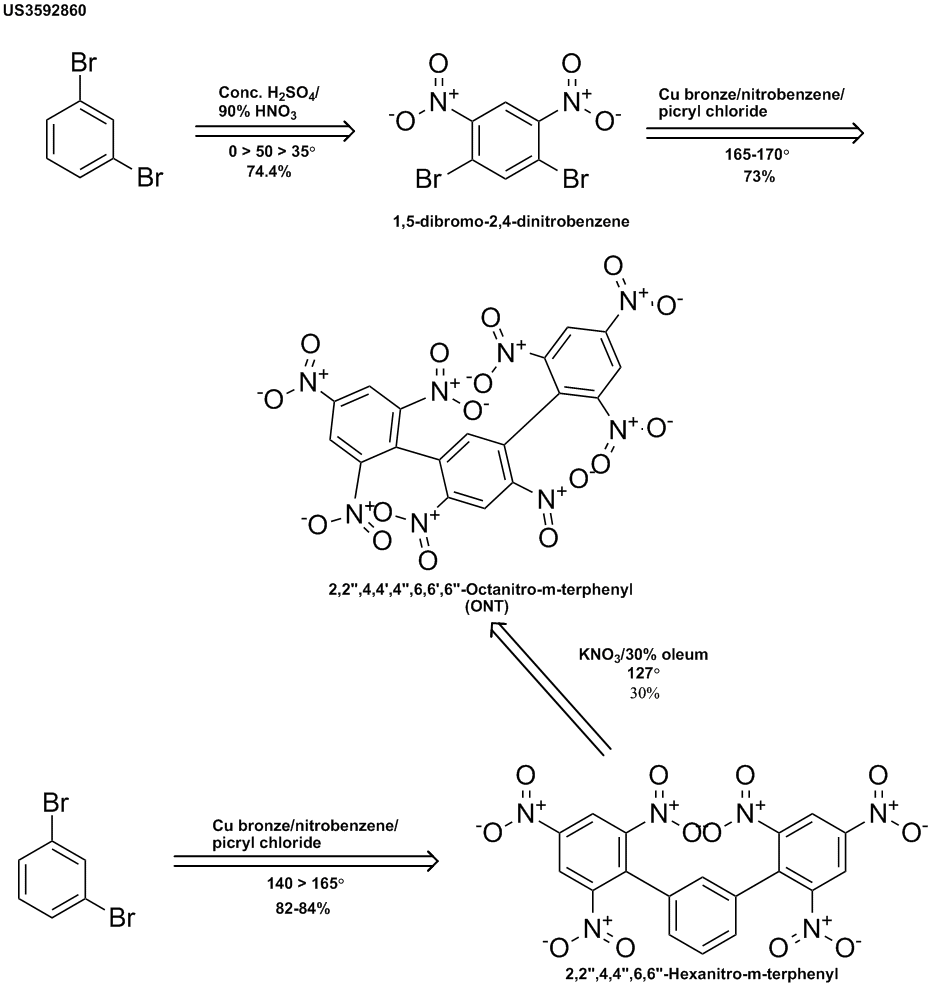
This explosive was prepared by the U.S. Navy & patented in 1971. (http://www.pat2pdf.org/patents/pat3592860.pdf) 1,3-Dibromobenzene was nitrated & then reacted with picryl chloride under Ullman conditions to
give the title compound. It was also prepared from the unnitrated dibromobenzene to give the hexanitro-m-terphenyl, which gave the same explosive on
nitration but with much lower yield.
It was stated that ONT could function well as an explosive at high temperatures. It was further stated that its crystalline nature made it useful in
high temperature plastic bonded explosives. It was further described as being thermally stable, sensitive to impact & capable of delivering a
great deal of energy (no quantitative data given).
As a side note, ONT was compared to NONA (2,2',2",4,4',4",6,6',6"-Nonanitro-m-terphenyl) whose preparation was disclosed in a different patent. It was
stated that the performance on ONT did not differ appreciably from that of NONA. The structures of both ONT & NONA were shown in the ChemDraw
graphic but the NONA structure was cut off during the upload.
[Edited on 28-7-2008 by Ritter]
ONT
Formatik - 7-8-2008 at 22:38
My relative strength estimate: 114% of TNT, by averaging the results of two methods. And for a related compound,
2,2',2",2"',4,4',4",4'",6,6,6",6"'-Dodecanitroquatraphenyl, molecule shown below, the estimate was 103% of TNT.


