
Picric-A - 28-8-2008 at 03:24
Has anyone had any experience with these?
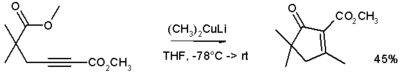
From looking at the synthesis some dont look too difficult to make however i dont know the nature of the reagents involved...:
'Lithium dimethylcopper (CH3)2CuLi can be prepared by adding copper(I) iodide to methyl lithium in tetrahydrofuran at −78 °C.'
Methyl lithium can be prepared as follows:
In the direct synthesis, methyl bromide is treated with a suspension of lithium in diethyl ether.
2 Li + MeBr → LiMe + LiBr
I think in the synthesis the tetrahydrofuran can be swapped with diethyl ether as i read its properties are very similar.
[Edited on 28-8-2008 by Picric-A]
Sauron - 28-8-2008 at 03:35
Organolithium reactions require exceptionally rigorous drying of materials and glassware and equally expert technique. They are an order of magnitude
more difficult than Grignard reactions.
Do you think you are up to it?
Got a drying oven and a large cylinder or dry Argon? A glove box set up for anhydrous positive-overpressure?
No?
Pity.
ScienceSquirrel - 28-8-2008 at 03:37
Methyl lithium is reactive stuff.
Alkyl lithiums are far more air sensitive than Grignard reagents and a lot stronger bases.
THF is a much stronger ligand than diethyl ether due to the fact that the alkyl group is 'tied back' by being in a ring. A lot of this type of
chemistry relies on the fact that THF has this extra complexing ability so you cannot just do a simple replacement.
[Edited on 28-8-2008 by ScienceSquirrel]
Picric-A - 28-8-2008 at 05:29
aah kk so bloody impossible to perform, bloody expensive to do, dangerous reagents...
ok get the picture.. lol 
panziandi - 28-8-2008 at 08:56
You should be able to get THF if you are in the UK cheap enough from an online supplier U2U me for details. If you have lithium I expect you have
sodium? Well even if you don't drying the THF wouldn't (or shouldn't) be a problem. Argon... not a problem either. The tricky bit would be -78*C
unless you have access to dry ice but ice-calcium chloride hexahydrate system cools to something quite low IIRC. That temperature is needed as
organolithiums form dimers and tetramers etc and have low half lives. So long as you don't hang around you should be ok...
not_important - 28-8-2008 at 18:16
It's difficult to keep low temperatures for long using ice-salt baths unless you go through a lot of the salt and ice, and/or start with a large and
well insulated bath. Pre-cooling the reagents, first near 0 C then with a less extreme choice of salt-ice helps.
But if you see reaction times of several hours, dry ice is your friend. It's also useful for drying the gas for the inert blanket, -78 C drop the
water level rather low (the outgassing off LN2 is better, but most corner shops don't stock it)
Klute - 29-8-2008 at 05:35
in my experience, inert atmospher is really required with organo lithiums... ANd it's certainly not the kind of reaction one cahn learn to perform
alone at home. You need to master schlenk technic, and have a few O2-sensitive reaction behind you to realize how quickly O2 can enter a reaction
setup, and be prepared for the many things that can go wrong.
I once had a fire with n-BuLi as the addition funnel stopcock wasn't tigntened enough, and over 20min a small drop form, as soon as it was in contact
with the atmospher it spontaneously caught fire. Hopefully, the addition was nearly over, and some Liq. N put it off easily. Imagine if the addition
funnel had been full and ht eseptum had blown off from the pressure caused by the heat?
Basicly, I think these reactiosn are the kind you ahev to learn to do in a academic or proffessioanl lab. No matter how much you read on the subject,
the main point in experience and deatils, whichonly someone with a few years practice behind can give. The risks can be high especially in amateur
settings, as often the security conditions aren't ideal (solvents not in fire-proof fume cupboard, lack of personel, fire doors.. etc)
There's lot of interesting reactiosn that can be done without directly jumping to organo lithiums and schlenk technic in general.
panziandi - 29-8-2008 at 09:11
I have prepared and used aryllithiums at home in THF, ok I used a schlenk flask under argon and manifold setup. I only performed it on a max 0.1
scale. But I'd recommend using Grignards a few times before you move onto simple organolithiums.
Klute - 29-8-2008 at 13:36
I totally agree with you. Grignars ar e nice in that they are very versatil, and have a historical aspect also, and are a good way on practicing
driied solvents, handlign and preparation, inert atmsopher, ro at least CaCl2-guards, and sometimes delicat intitiation.
I suppose you have already soem academic training, notable on hwo to sue schlenk lines and inert atmospher in general. I try to explain thsi by
message, btu obvioulsy only direct handlign in front/with someone can teach such manipualtions.
If trying the autodidacte way, I think less hazardous reagent shoudl be used, in the sense where a little H2o or O2 can destroy them, but tey cna't
put your house on fire  So that people can get a grip at how sensible each
manipualtion is, btu with space for mistakes.
So that people can get a grip at how sensible each
manipualtion is, btu with space for mistakes.
Is your manifold setup commercial glassware, or some kind of DIY setup?
I've been planning on builinding a manifold from two distribution ramps, I have the solvent LN trap, but I don't have an adequate pump for now.
Waiting to have a little more money to spend.
panziandi - 29-8-2008 at 14:02
I'm a biochemist by academic training (uni) and have not been trained on Schlenk per se, I read books and practised (chromium (II) chloride gives a
safe idea of how to handle things first few times as it changes from blue to green if you mess up and let air in!). I had a simple single bank
manifold when I was about 16 but had used inert atmosphere by just passing Ar through a steam leak into a setup before that. Now I have a dual bank
manifold! The manifold has three B14 ports each linked to the vacuum and inert lines, both with their own teflon taps, the vacuum line has a
connection to vacuum gauge and a ball joint for connecting to a cold trap (with isolation teflon tap). All teflon taps are High Vac rated and the
construction is from thick walled borosilicate. I also lack a descent pump but purging with Ar is fine and is good for transfers too, I connect it to
my water aspirator for use with my rotovap. My manifold was made by my local glassblower, check my site for contact details for him he is very
reliable.
Klute - 29-8-2008 at 16:34
I have a very similar setup at work. The single-stage manifold (distribution ramp) was given to me by a collegue.
I also have a very good relationship with a local glassblower, from which I get part of my reagents too. they are a very kind team, with who I share
all my reactions and projects, they are particularily fond of the nice smelling phenylbutanones and esters.
Nice site, BTW!


 So that people can get a grip at how sensible each
manipualtion is, btu with space for mistakes.
So that people can get a grip at how sensible each
manipualtion is, btu with space for mistakes.