Originally posted by woelen
The purple solid quickly turns brown and when the acid is
heated, I obtain a reddish brown precipitate in a colorless liquid. To this, I added water, dropwise. At a certain point, the very fine precipitate
dissolves and a deep purple and clear liquid is obtained. When more water is added, then the color of this solution shifts from purple (like
permanganate) to reddish purple and from there to red (something like shown in the picture above of the erlenmeyer) and on addition of even more
water, the solution becomes turbid and a dark brown precipitate is formed.
So, in fully concentrated H2SO4, manganese(III) seems to exist in the form of a reddish/brown solid. Is this Mn2(SO4)3? On moderate dilution of the
acid, a deep purple solution is obtained. This is not plain Mn(3+) in solution, but must be some sulfato-complex of Mn(3+), a possible option could be
[Mn(SO4)2](-). On even stronger dilution, the color shifts to red/brown and then aqueous Mn(3+) is obtained (as shown in my testtube picture in a
previous post in this thread). When dilution is even stronger, the Mn(3+) hydrolyses to Mn2O3.
] |





 .
.









 <sub>3</sub> forms alums with Rb and Cs, albeit not very stable ones. I wonder if it would be possible to pull a potassium alum from the
purple solution... It'd be nice to have a solid Mn(III) bearing compound...
<sub>3</sub> forms alums with Rb and Cs, albeit not very stable ones. I wonder if it would be possible to pull a potassium alum from the
purple solution... It'd be nice to have a solid Mn(III) bearing compound...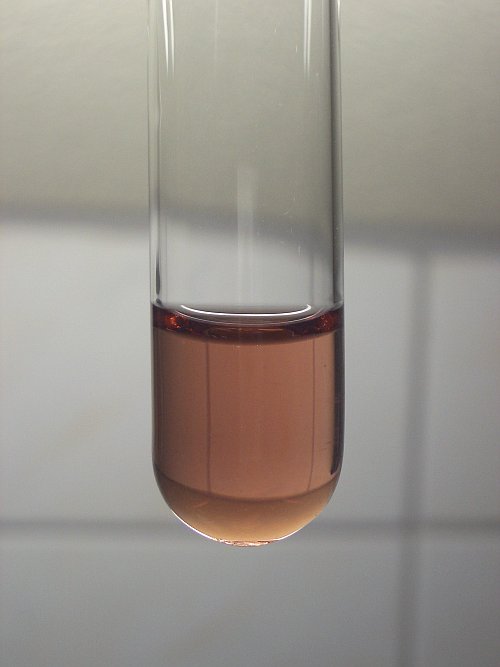




 + water ---> Mn<sub>2</sub>O<sub>3</sub> or
Mn<sub>3</sub>O<sub>4</sub>
+ water ---> Mn<sub>2</sub>O<sub>3</sub> or
Mn<sub>3</sub>O<sub>4</sub> makes me think that the violet
colour is indeed simply Mn<sub>2</sub>(SO<sub>4</sub>
makes me think that the violet
colour is indeed simply Mn<sub>2</sub>(SO<sub>4</sub> <sub>3</sub>.
<sub>3</sub>. <sub>3</sub>.
<sub>3</sub>. <sub>3</sub> based alum, with K, Rb or Cs...
<sub>3</sub> based alum, with K, Rb or Cs...

 <sub>2</sub><sup>-</sup>, huh?
<sub>2</sub><sup>-</sup>, huh? <sub>2</sub><sup>-</sup>.
<sub>2</sub><sup>-</sup>.

