3 - Nitroperchlorylbenzene
O2NC6H4ClO3
"McCoy, G., Chem. Eng. News, 1960, 38(4), 62"
The nitration product of perchloryl benzene is explosive, comparable in shock-sensitivity with lead azide with a very high propagation rate.
1,1,1,3,5,5,5 - Heptanitropentane
(O2N)3CCH2CH(NO2)CH2C(NO2)3
"Klager, K. et al., Propellants, Explos., Pyrotech., 1983, 8, 25-28"
This explosive compound has a +12% oxygen balance so can function as an oxidant.
1,1-Diazidoethane
(N3)2CHCH3
"Forster, M. O. et al., J. Chem. Soc., 1908, 93, 1070"
The extreme instability and explosive behavior of this diazide caused work on other gem-diazides to be abandoned.
Aluminum tetraazidoborate
Al[B(N3)4]3 or AlB3N36
"Mellor, 1967, Vol. 8, Suppl. 2, 2"
A very shock sensitive explosive, containing nearly 90 wt% of nitrogen.
|
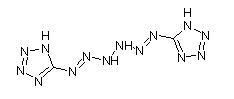


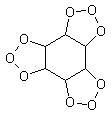
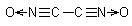
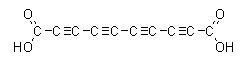



 Many people have
paraformaldehyde, HNO3 and H2SO4, I guess.
Many people have
paraformaldehyde, HNO3 and H2SO4, I guess. 

