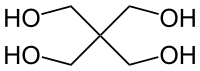
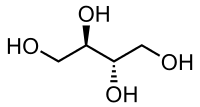
Erythritol contains 4 carbons, one fewer than pentaerythritol. . .
But the difference in performance between ETN and PETN is slight and erythritol can be nitrated by KNO3/H2SO4 mixtures, whereas PETN preparation requires strong, white HNO3.

Quote: Originally posted by hissingnoise  |