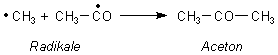Kinetics and mechanism of thermal decomposition of lithium, sodium, and barium acetates. Yakerson, V. I.; Rubinshtein, A. M. Kinetika i Kataliz, 2
(1961), 172-178.
CA abstract: Prepn. of ketones by thermal decompn. of Li, Na, and Ba acetates and prepn. of CH4 by thermal decompn. of NaOAc are studied.
Prepn. of ketone from NaOAc (at 419-74) and LiOAc (at 352-415°C) occurs in the melt and proceeds by 2nd-order kinetics. Ba(OAc)2 decomps. in the
solid phase in accordance with the topokinetic Erofeev equation (CA 41, 4027c). The relation between the rate consts. and temps. is described by the
Arrhenius equation. The energy of activation (E) and preexponential factors were calcd. E of prepn. of ketones from the acetates agree with E of
vaporphase prepn. of ketone from AcOH on carbonates of the corresponding metals. This fact holds good also in the prepn. of CH4 from NaOAc and from
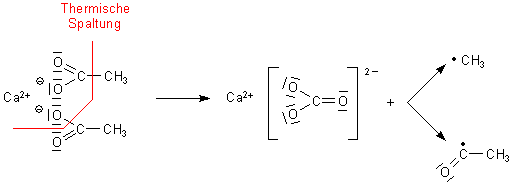
AcOH on Na2CO3. The exptl. results and literature data verify the concept of the formation of a cyclic 4-membered activated complex and of the mol.
mechanism of ketone prepn. A scheme is suggested for the prepn. of CH4 from NaOAc with participation of H2O included in the activated complex. A
compensation effect exists for both types of decompn. of the studied acetates.
...
General Procedure: The dry distillation was carried out in a 50 mL round-bottomed flask fitted with a 10-cm Vigreux column connected to a
microdistillation apparatus and the gas outlet was connected to a gas burette. The flask was heated with a hemispherical heating mantle and the
reaction time was started when 350 °C reaction temperature had been reached (after ca. 25 min). Adipic acid (10.0 g, 68.4 mmol) was placed in the
flask together with a magnetic stirring bar and the corresponding amount of base. The heating was started and the progress of the reaction was
followed by measuring the gas evolution. When no gas was produced anymore, the reaction was stopped, the two phases separated and the organic layer
analysed by GC analysis, GC-MS and NMR spectroscopy. The purity of the cyclopentanone was in all cases higher than 99%. Traces of unchanged adipic
acid could be detected by 1H NMR spectroscopy. In the case of the re-use of the residue, adipic acid (10.0 g, 68.4 mmol) was placed in the flask
together with NaOH (496 mg, 12.4 mmol) and distilled until gas production ceased. A new 10.0 g portion of adipic acid was placed in the flask and the
distillation continued. Over seven cycles 36.4 g (90%) of cyclopentanone was obtained as a colourless liquid from 70.0 g of adipic acid. from Eur. J.
Org. Chem. (2004) 2036-2039 |