| Quote: |
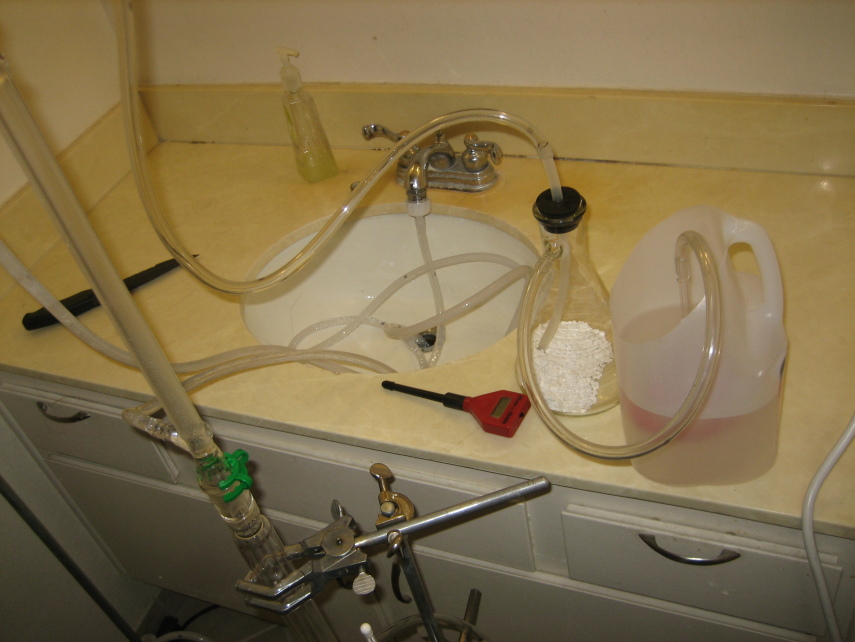
| Originally posted by MagicJigPipe ... I guess my whole point is. Why is Hg vapor so great and why should I spend the extra time and effort to get one? ... |
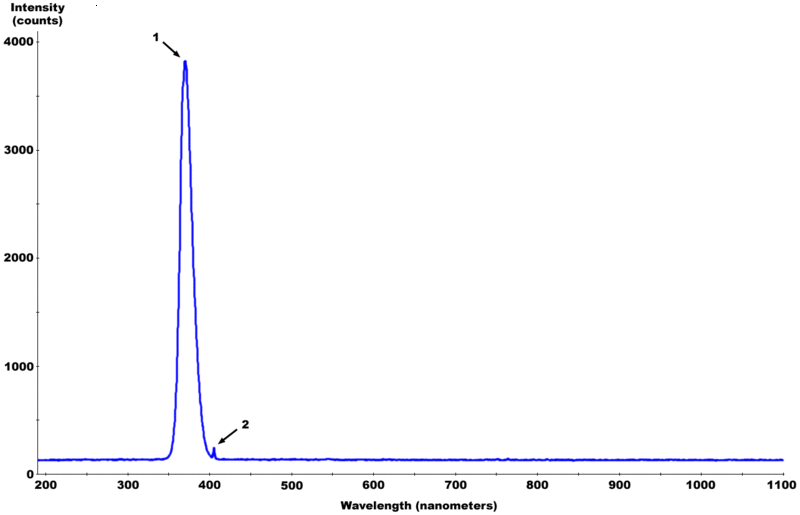
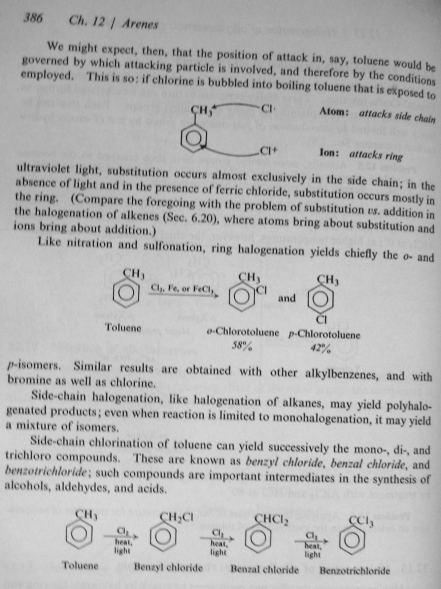
Because as I said earlier, Chlorine radical reactions are triggered by light in the 300 to 380 nm range, with some effectiveness on up to 430 nm or so. The phosphors in a fluorescent lamp convert most of the UV to wavelengths longer than 550 nm
http://commons.wikimedia.org/wiki/Image:Fluorescent_lighting...
mercury spectrum
http://www.lamptech.co.uk/Documents/M3%20Spectra.htm