
andre178 - 15-4-2010 at 14:13
Hello, I'm actually a newbie chemist, so if this question belongs to beginnings, admins plz feel free to move it.
I am making the malonyl dichloride ClCOCH2COCl via malonic acid and thionyl chloride, which generates sulfur dioxide and HCl on top of the product.
SO2 will bubble out, and HCl is gonna become problematic. Can anyone help out with how much heat I need to supply, and how I can isolate the product
from HCl?
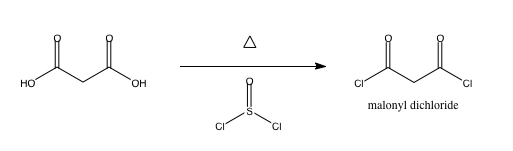
not_important - 16-4-2010 at 01:56
The HCl will be the anhydrous gas, and much of it will exit with the SO2.
A preparation of malonyl chloride is included in http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=cv4...
A bit of a advanced starting point for a newbie who didn't even check OS or Vogel first.
