
Anders Hoveland - 2-7-2010 at 13:08
I reacted hydroxylamine with a mix of mono and dinitro phenol. The nitro-phenol was prepared as described elsewhere in this forum. The NH2OH was made
from CH3NO2 and HCl pool muriatic acid. I think there may have been some bicarbonate contaminants from the neutralization.
I am not completely certain that the nitro-phenol condensed with the NH2OH, instead of just forming a salt.
In a threaded metal pipe, the presumed (NO2)PhNHOH was placed with solid KOH pellets. Plastic caps were placed on both ends (the metal ones were
really expensive) and the central part of the pipe was placed over a small sawdust-vaseline firepit and abandoned. An explosion was heard quite some
distance away. One of the caps blew off, propelling the pipe into some bushes. The inside could not be washed out, as it was obviously insoluble, but
some oily droplets came out. The inside was chiseled out, and more oily droplets were obtained. This may have been the intended product: benzene
furoxan.
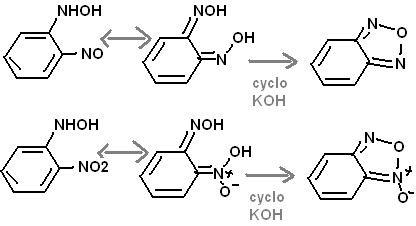
The furozan of benzene has resonance with 1,2-dinitroso benzene, so reduction with bisulfite should be possible to make 1,2-di[hydroxylamino] benzene.
The oily liquid droplets were placed in a NaSO3H solution. The droplets seemed to get smaller, but were unable to completely dissolve. The solution
was evaporated and crystals precipitated out, but these crystals did not become carbonized on exposure to flame. After removing crystals, and further
evaporating, I was unable to isolate any benzene crystals. But the oil obtained to begin with was so little, it might not have been enough to work
with.
The solid chunks that had been removed from the pipe, readily dissolved in water, leaving a few more oil droplets that neither seemed to float to the
surface, nor sink to the bottom.
2-Nitro, N-hydroxy analine should share resonance with the 1- ==NOH, 2- ==N(O)(OH) of benzene. This likely cyclized to some extent when under heat,
pressure, and dehydrated by anyhrous fused KOH.
But again, I cannot be sure that the condesation product of
(NO2)C6H4NHOH was even formed in the first place. I tried to crystallize nitro phenol out by putting into the freezer, thinking NH3OH(NO3) would be
much more soluble, but I it had to be really cold to freeze out, and what solids could be frozen out reacted with bicarbonate, and I was then able to
get crystals that did not carbonize when burnt but did oxidize charcoal when molten (NaNO3). This indicated that the original crystals frozen out
contained an HNO3 as a salt, and that they were not merely nitrophenol alone.
If condensation had not taken place, I would have expected nitrophenol to crystallize out first, and hydroxylamine nitrate to stay in solution until
the end.
Let me suggest that if this is repeated, the furoxan could be nitrated to dinitro benzene furoxan, formula C6H2N4O6, and that this could be reduced
with bisulfite (that would spare the nitro groups) to 1,2-di[hydroxylamino],3,5-dinitro benzene, which would form an energetic salt with HClO4
(it was kind of obvious were this paragraph was headed,
just assume whenever you see a NHOH group on one of my compounds, it is probably going to react with perchloric acid before the synthesis is over)
Anders Hoveland - 5-7-2010 at 22:56
To expand on why the furoxan is expected to form, let me quote from the prepublication section:
"Diaminofurazan is formed from the base catalysed dehydration and cyclisation of diaminoglyoxime by aqueous sodium[23] or preferably potassium
hydroxide[24,18] at 180°C. Recently a convenient microwave mediated synthesis has also been reported[22]."
Thus two adjacent oximes can afford a furaxan.
The picture should clarify the reasoning.
[Edited on 6-7-2010 by Anders Hoveland]
Thiofurazan
AH-Poster - 19-10-2010 at 14:05
The reaction of sulfur nitride with acetylenes was carried out in refluxing toluene to give 1,2,5-thiadiazoles as a major product. "Preparation of
3,4-disubstituted 1,2,5-thiadiazoles by the reaction of sulfur nitride with acetylenes" by Shuntaro Mataka
This is basically a furzan ring, with sulfur substituted for the oxygen atom.
http://www.google.com/imgres?imgurl=http://upload.wikimedia....
"thio-furazan" (see link above for picture)
Not sure if this might be useful to any readers.
