This is amazing, there are no photos of liquid ozone on the entire Web, and I haven't seen it in books, either.
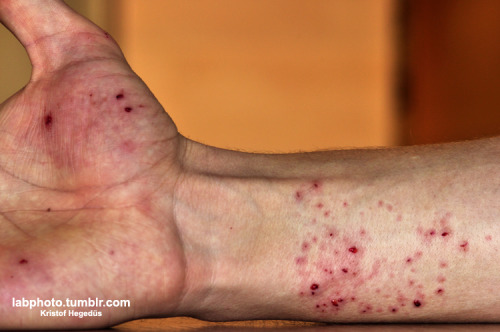
I'm sorry about the mishap, I hope your hand is ok, but well done! This is the real thing. Just... wow!
If that's from an ozonizator, it's not pure ozone, am I right? After all, LN2 should've freezed it.
Or you've managed to remove excess oxygen and nitrogen from the air? Have you fed the machine with pure oxygen?
[Edited on 27-8-2012 by Endimion17]









 I don't have the equipment to do so, though.
I don't have the equipment to do so, though.