
Quote: Originally posted by elementcollector1  |
| What about catalyst? What comes to mind is that the K forms, but when the Mg runs out the catalyst turns right around and starts consuming the K metal [...]. |
My thoughts exactly. Then again, I could be very wrong,
[Edited on 9-3-2013 by Mailinmypocket]

 .
That should really rule out any temperature problems.
.
That should really rule out any temperature problems.



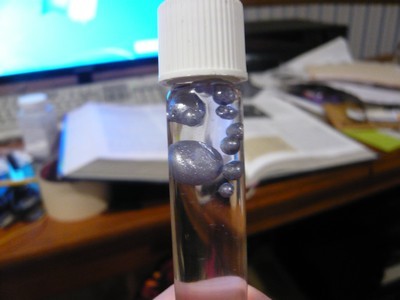

 very nice though, good job! Ill have to read over your last couple posts in
detail when I have time, but this is very promising!
very nice though, good job! Ill have to read over your last couple posts in
detail when I have time, but this is very promising! 
