
JCM83 - 13-1-2011 at 13:18
So I dissolved sodium chloride (table salt) in acetone by heating it to boiling and then allowing it to cool. I believe that that had a solution of
sodium and chloride ions in acetone.
I then put in two copper electrodes- just the stripped ends of the copper wires- under a fume hood.
I expected to develop chlorine gas and sodium metal. What I got instead, was that the anode bubbled up some gas, and the cathode appeared to
<i>flake and fall apart</i>. Shortly thereafter my acetone solution appeared pale green.
Question- what the heck happened? Did I generate copper chloride solution in the acetone, and the sodium did indeed electroplate but was then oxidized
by the acetone generating, like... hydrogen gas and sodium acetate? So that I'm left with a solution of copper chloride and sodium acetate?
What's the deal man?
[Edited on 13-1-2011 by JCM83]
not_important - 13-1-2011 at 14:56
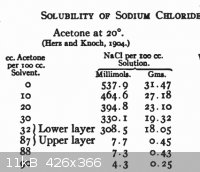
The solubility of NaCl in acetone is quite low, see the Finkelstein reaction and the attached chart - which is for water-acetone mixtures.
The copper is attacked by the chlorine. The hydrogens of the acetone are acidic, sodium will react with them releasing hydrogen and forming
CH3C=OCH2(-) Na(+). This is not stable under the reaction conditions, it can react with the copper ions, and the organic can undergo condensation to
form various compounds that is sometimes known as glop, but you'll not have much because of the low solubility of NaCl. You might get some
reduction of acetone to the alcohol. Also the copper ions can plate out instead of sodium.
JCM83 - 13-1-2011 at 17:59
Ah, I see, thank you. I actually added a few mls of water to get the sodium chloride to dissolve and I think that's what was preventing me from
getting anything useful.
Do you know of a way I can successfully synthesize alkali metals in a simple lab like I've got? Plenty of glassware and reagents, no advanced stuff
unless I kludge it together myself, and my background's bio and neuro rather than practical lab chem like this.
Sedit - 13-1-2011 at 18:01
I think the search engine would be more then helpful here as there is a VERY active topic right now on the low temperature synthesis of Potassium
using Magnesium on KOH in an inert solvent. According to the patent this works for a variety of alkali metals.
bquirky - 13-1-2011 at 21:31
I tried a similar thing once. I dissolved a small amount of NaOH ito some Ethanol with a very small amount of water and attempted to electrolyse it I
ended up with a dark amber coloured gloop that had an extremely noxious odour.
I re ran the experiment using aluminium chloride dissolved in ethanol and wound up with no noticeable change to the electrolyte except some
evaporation and a grey powder on the cathode.
When scraped off this grey powder fizzed in a sodium hydroxide solution so it may well have been aluminium that plated out.
I started playing with some choline chloride ionic liquids but didn't get very far.
Eclectic - 13-1-2011 at 23:37
Electrolizing 25% acetone/water saturated with sodium chloride might be worth trying as a means to make chloroform. Maybe with graphite electrodes.
[Edited on 1-14-2011 by Eclectic]
