
B.D.E - 5-8-2019 at 21:24
Hey there,
First thing first, it's seems like an awesome forum and I'm very glad to have found it 
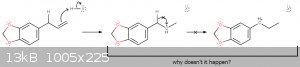
To the point, I have a theoretical question about the halogenation of Safrole using Hydrogen halides.
From what I've learned on lectures, benzylic carbocations are usually much more stable then alkyl carbocations. if so, why there's no hydride shift
taking place in such a reaction?
I get that the simplest answer is "because it just doesn't happens", but if anyone have some satisfying rationalization for that or insights to share,
it would be swell.
[Edited on 6-8-2019 by B.D.E]
[Edited on 6-8-2019 by B.D.E]

