Cou - 20-11-2020 at 14:07
https://doi-org.libproxy.utdallas.edu/10.1021/ol020071y
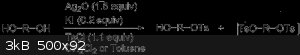
The reaction of symmetrical diols and oligo(ethylene glycol)s with a stoichiometric amount of p-toluenesulfonyl chloride in the presence of silver(I)
oxide and a catalytic amount of potassium iodide led selectively to the monotosylate derivatives in high yields. However the ditosylate is still
formed in small amounts. Fractional distillation or chromatography could separate the monotosylate and ditosylates.
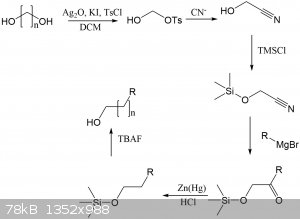
I have attached a synthetic route where this monotosylation could theoretically be used to extend the length of any alcohol by any length, depending
on the length of the diol. Several lengths of diols and dicarboxylic acids (which can be reduced to diols) are commercially available.
Imagine being able to make some wacky alcohol like this. NOw it would be possible, by making a tertiary alcohol and then extending it. No real world
use I can think of, except it's cool:

This would be a fun project for another time, but there's so many steps here that yields would be low. Some of the steps could be combined into
one-pot procedures, so fewer purifications/extractions are required.

[Edited on 11-20-2020 by Cou]
[Edited on 11-20-2020 by Cou]