yobbo II - 1-12-2020 at 16:50
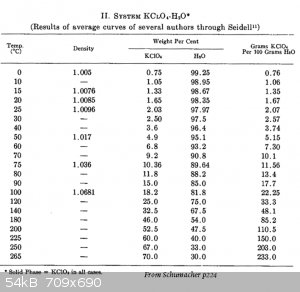
Attached is a table from The Perchlorates by Schumacher (book).
It would appear that you can heat solutions of potassium perchlorate solutions all the way up to 265C.
Is this true or am I missing something?
Yob
MidLifeChemist - 1-12-2020 at 17:19
Well a saturated solution of KClO4 will boil at a temperature between 100 and 120 degrees. There is a formula to calculate the exact boiling point,
google boiling point elevation of electrolytes. So yeah, your missing something 
B(a)P - 1-12-2020 at 19:03
Pressure I suspect.
woelen - 2-12-2020 at 07:25
At atmospheric pressure you'll get dry KClO4 a few tens of degrees above 100 C. If you continue heating to 265 C you get dry KClO4. If you go higher,
then the KClO4 decomposes, giving KCl and O2.
yobbo II - 2-12-2020 at 11:06
It's just that the pressure was not mentioned in the table or the text around the table.
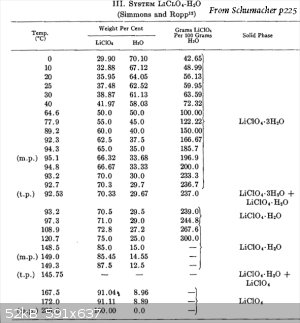
Here is another table showing Lithium perchlorate. It appears to be saying something similar, ie. you can heat the solution way up to the melting
point of Li Perchlorate.
(Li perchlorate actually has a melting point without decompositon unlike other perchlorates.)
I would be inclined to imagine that if you held a solution of Li perchlorate at 150C for a long period of time at STP, then all the water would dry
off.
t.p. is noted in some places. What is that. Also m.p. (melting point) is noted. Melting point of what? The last row I can see what m.p. means (the Li
Perchlorate).
Perhaps I need to go back to Simmons and Ropp!
Yob