
Boffis - 1-2-2021 at 12:06
I am looking at preparing acenaphthalone. It can be prepared from both acenaphthene (C12H10) and acenaphthylene (C12H8) but I wonder if it could be
prepared from naphthalene via Friedel Craft acylation/alkylation from naphthalene and chloroacetyl chloride.
There is an old German patent D230237, that prepares this compound via 1-naphthylacetyl chloride and AlCl3 (Friedel Craft reaction). The preparation
of acenaphthenequinone from oxalyl chloride and naphthalene gives a very poor yield (see Staudinger et al; Helv. Chim. Acta; 1921 p334). Acetyl
chloride give mainly 1-acetylnaphthalene so chloroacetyl chloride should give the chloroacetylnaphthalene but would it then cyclotize to the ketone?
Any thoughts?
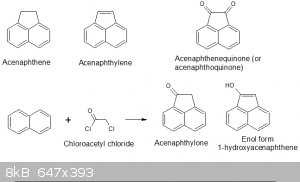
Alternative: the best way to 1-naphthylacetic acid is??
Edit: just spotted the thread on the Willgerodt reaction, from1- acetylnaphthalene looks like a more promising route.
[Edited on 1-2-2021 by Boffis]
