
Lion850 - 23-8-2021 at 18:30
When I recently posted on making metal picolinates, some members pointed out that nicotinic and picolinic acid are isomers and wondered whether the
colors of the corresponding metal salts would differ. I got some food grade nicotinic acid and started making metal salts to see.
Copper nicotinate: I first tried to react copper carbonate direct with a solution of nicotinic acid, and while there was a reaction (bubbling) it
slowed down quickly and was difficult to see if the reaction went to completion. I thus changed tact and proceeded as follows:
- 4g NaOH dissolved in 50ml water (pH ~14).
- Start adding small amounts of nicotinic acid, waiting after each addition until the solution was completely clear and checking pH each time.
Stoichiometry said around 12.5g will be required, but I was not sure if the nicotinic acid is anhydrous and whether the NaOH was completely dry.
- Once 11g of nicotinic acid was added the pH dropped for the first time, to ~9.
- After 12g was added the pH was ~6 and I took this as completion of the reaction. At this stage the solution was completely clear.
- 13g copper nitrate was dissolved in 35g water. The clear blue copper nitrate solution was added to the sodium nicotinate solution while stirring.
Solutions before mixing:

- A sky blue suspension immediately formed:

- After 15 minutes stirring the solution was filtered. The filtrate was pale blue with a sky blue remainder. The remainder was washed once in the
funnel with water:

- The remainder was left out to dry overnight, and then put in the sun for 6 hours the next day. The result was a hard blue product:

- This was broken into pieces and placed in a bottle. 18.1g was recovered, which would indicate the copper nicotinate to be a hydrate.

- Below is a comparison photo showing the copper picolinate and nicotinate. The picolinate has some purple to it, with the nicotinate being sky blue.
I do not know if the different color is purely a property of the structure of the isomer, or whether the fact that the salts were made different is
also a factor.

Chromium to follow.
draculic acid69 - 23-8-2021 at 22:27
Cool looking colours. Both Look like they were made from smurfs
Lion850 - 24-8-2021 at 00:02
Hahaha smurfs....I like it 
Chromium nicotinate:
- Sodium nicotinate was made as per the first post, by adding nicotinic acid to a solution of NaOH. Similar quantities were used.
- 13g Chromium Nitrate nonahydrate was dissolved in 30g water, giving a dark solution:

- Add the chromium solution to the sodium solution while stirring.
- Initially it appeared that nothing was happening. After a few minutes I noticed that stirring had stopped, and found that the bottom of the beaker
now had a sticky layer that was preventing the stir bar from moving.
- I freed the stir bar with a glass rod and by that point the sticky layer was becoming hard and breaking up. Stirring restarted and I noticed purple
crystals sticking to the beaker sides.

- After around 10 minutes of stirring the solution was all purple with crystals swirling around:

- The solution was filtered. The remainder was washed in the funnel. The filtrate was pale purple and the remainder a dark purple:
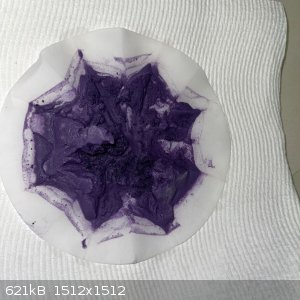
- The remainder was transferred to an evaporating dish and left on the bench overnight, followed by 6 hours in the sun the next day. The result was
15.9g of light soft purple powder:

- There is quite a difference in color between chromium picolinate and nicotinate!

Cobalt, nickel, and iron still to come.
Lion850 - 25-8-2021 at 23:39
Iron:
Similar procedure as above: first make sodium nicotinate and then a double displacement with an iron salt. Just to see if there is any difference I
did the reaction twice, first with iron chloride and then with iron nitrate. In both cases the results were exactly the same.
- Iron solution and sodium nicotinate solution (in this case the iron chloride)

- After mixing solids appeared while stirring

- After a few minutes stirring the solution was a uniform brown suspension.

- Filtering gave a brown remainder and almost clear filtrate.
- One part of the remainder was dried on a steam bath and the other in the sun. Both methods have a fine brown powder (after crushing).

- The color of the iron nicotinate is quite different from the picolinate.

Observations:
- The nicotinates are generally slower to gravity filter than the picolinates, but this iron nicotinate was the worst - I had to leave it filtering
overnight.
- The brown product is readily soluble in HCl, giving a yellow solution.
Cobalt and nickel to come.
woelen - 26-8-2021 at 23:39
Interesting observations. What surprises me is that the chromium compound becomes so light after drying. Are you sure that the dark streaks on the
glass in your first photo of the chromium compound are crystals. I have the impression that it is more like a gel, which breaks up in smaller
particles on stronger stirring giving the bright purple homogeneously looking suspension of the second photo.
You also mention that the brown product (ferric nicotinate) dissolves in HCl, giving a yellow solution. This yellow solution most likely is due to
formation of the yellow FeCl4(-) complex (any iron(III) salt gives yellow solutions in HCl). Try dissolving it in dilute sulfuric acid or dilute
nitric acid (appr. 2M sulfuric acid or 3M nitric acid is a good starting point). I expect you to get a nealy colorless solution in that case.
Lion850 - 27-8-2021 at 20:46
Hi Woelen, yes wrong choice of word. Probably streaks of the same sticky stuff that also formed in the bottom of the beaker, then hardened and became
the fine suspension. Will try as suggested with the brown ferric nicotinate. The frequent observations of what happens with HCl is purely because HCl
is cheap and readily available as pool acid and basically my go-to for cleaning the beakers and evaporating dishes. With a completely unproven
observation that I have not had a cold or flu for more years than I can remember which I think may have something to do with getting an accidental
sniff of HCl fumes at least once a week! (don't take this as medical advice haha).
Edit: I read my post on iron picolinate again and that also gave a yellow solution with HCl.
[Edited on 28-8-2021 by Lion850]
Lion850 - 30-8-2021 at 00:42
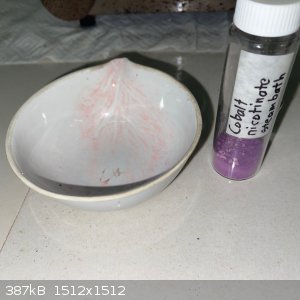
Cobalt nicotinate turned out to be quite interesting!
- A solution of sodium nicotinate was made as per the previous posts, and a stoichiometric solution of cobalt nitrate hexahydrate added. After some
stirring a pinkish suspension was present:

- The solution was filtered, both the filtrate and the remainder was a similar color:

- The remainder was left in the sun for some 5 hours; this was the result:

- I scratched out some of the product to transfer to a vial and then got called away. When I collected the evaporating dish from the sun an hour later
some of the material had turned purple where it was thinly layered. Sorry I did not take a photo.
- I then squashed the soft pink lumps into more of a powder and placed 5 gram on a steam bath:

- It soon started to turn purple. I then placed a watch glass on the evaporating dish and observed condensation, showing the material was releasing
water:

- After some 3 hours on the steam bath the bulk of the material was now purple, apart from a few bigger lumps that was still pink. Below shows both
the original pink salt as well as the steambath-heated purple salt.

- I scratched the purple salt out of the dish while still hot and transferred it to a sealed vial.

- At the end of the day the leftover in the dish was still purple but by the next morning it had turned back to pink, while the material in the sealed
vial was still purple.
- I suspected the original pink filtrate to be a hydrate. Heating it up on the steam bath (or even direct sun) causes loss of water and change of
color to purple. But if the purple salt is left exposed to atmospheric moisture AND the ambient temperature is cool enough it seems to absorb moisture
and turn pink again.
The 5g of pink material resulted in 4.1g of purple material after being heated on the steam bath. This indicated a tetrahydrate. I then googled
"cobalt nicotinate hydrate" and indeed found this paper: "The Crystal Structure of Cobalt( 11) Nicotinate Tetrahydrate: a Non-classical Zwitterion" by
By A. ANAGNOSTOPOULOS, 31. G. B. DREW, and R. A. WALTON. This paper also mentioned the salt having a pink color.
When I made cobalt picolinate I got a similar pink color. But it did not change when dried on the steam bath. I need to look into this more to see if
the pink cobalt picolinate is also a hydrate. Family photo below.

Lion850 - 3-9-2021 at 20:50
Nickel nicotinate:
A solution of sodium nicotinate was made as before, by dissolving nicotinic acid powder in a solution of sodium hydroxide until the pH was near 7. A
green solution of nickel nitrate was then added to the clear solution of sodium nicotinate:

A light blue suspension formed:

After 15 minutes stirring the solution was filtered, and washed in the funnel. The filtrate was clear, with a light blue remainder. The remainder was
still quite wet even after sitting in the funnel overnight:

The wet remainder was then dried on a steam bath for some 3 hours by which time the weight loss had stopped. This was the result - if you look closely
you can see that there is some green color appearing apart from the predominantly blue:

10 gram of the blue nickel nicotinate tetrahydrate (presumably) was then heated to around 200 C on a steam bath for 2 hours (the final dry powder had
a similar color to nickel picolinate).

The blue powder soon started turning light green:

By the time it all appeared to have turned green approximately 2g weight was lost: 8g remained. These masses correspond well to the stoichiometry of
the equation:
(C6H4NO2)2Ni.4H2O = (C6H4NO2)2Ni + 4H2O
This picture showed the final tetrahydrate and anhydrous salts:

After learning that the cobalt and nickel nicotinates have both tetrahydrate salts and anhydrous with quite different colors, I heated up the copper
and chromium nicotinates obtained before on the sand bath to see what happened. But they both seemed to decomposed at around 200C.
The blue copper nicotinate decomposed to a black powder. There was no smell or fumes. This black powder reacts vigorously with drops of 70% nitric
acid with the release of red NO2 fumes.
The purple chromium nicotinate first turned grey and then black, again with no smell or fumes. This black residue did not show any immediate reaction
with nitric acid drops.
Below a family photo showing the pleasing metal picolinates and nicotinates colors. My daughter says these are "soft" colors 



