
phendrol - 27-4-2022 at 23:47
Hi!
I have a sample which is suppose to be ostarine of about 99% purity.
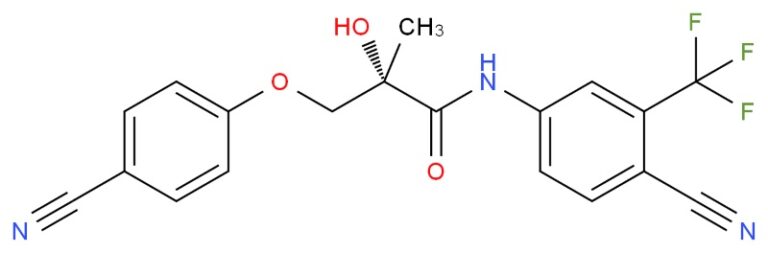
I want to verify it but lab expertise is very expensive. Can you guys give me some advice?
I was thinking to test the solubility and test for the presence of functional groups. Can someone suggest particular tests and reagents for the above
functional groups?
Any advice appreaciated!
Fery - 28-4-2022 at 03:32
Hi, not specific test but here I found m.p. 132-136 C
https://en.wikipedia.org/wiki/Enobosarm
Pumukli - 28-4-2022 at 11:17
I'd try to test for fluorine. Sample digested by alkaline hydroxide and then testing / measuring the freed fluoride ions.
phendrol - 28-4-2022 at 12:22
Any reference to the test for fluorine?
AvBaeyer - 28-4-2022 at 18:47
Fery's suggestion of the melting point is the first step to follow up on. If that checks you are ok if your purity estimate is valid. If the mp is a
bit off, recrystallize and try again. Your main problem with the mp is if the racemate melts differently from a pure enantiomer as you are dealing
with a compound having an asymmetric center. Do you know anything about the chiral purity of the sample? The melting points of the racemate (R/S)and
the resolved isomers (R or S) are likely known providing you with a challenge to find out.
There is no point initially to test for fluorine as that is much more difficult than suggested above. Testing for functional groups is not gong to be
easy. You need to consult a good lab manual on organic qualitative analysis which will provide you with procedures.
BTW, I would not mess with this stuff.
AvB

