
jan1234 - 13-12-2022 at 07:18
Hi,
i'm wondering if you could reduce both keto-groups in caffeine to their alkanes.
the reduction method i'm thinking of is mainly used to reduce secondary alcohols to alkanes, but i found some rather 'not properly reliant'
information on the internet, that it will also reduce ketones to alkanes (using hydroiodic acid, generated in situ using two elements in the presence
of water. i'm not sure if it's allowed to specify this procedure, since it's often used by hobby 'chemists' to get an illegal product).
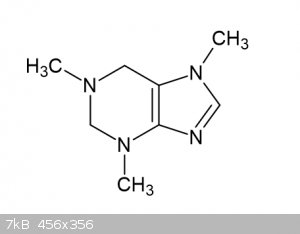
if it works and both keto-groups would be reduced, the product should be 1,3,7-trimethyl-3,7-dihydro-1H-purine (see attachment). it should be less
water soluble than caffeine. i found information about 6-methylpurine, but nothing on (hydrated) (tri)methylpurines.
my questions are:
1. has anyone ever tried reducing the keto-groups of caffeine?
2. has anyone ever come upon the proposed product?
3. can anyone with access to SciFinder try a quick search, if it has been mentioned in literature anywhere?
(4. are i am allowed to post the procedure to create the HI in situ (i'm sure some people here will know which one i mean)?)
Boffis - 14-12-2022 at 00:30
If you check out libgen there are a series of books on heterocyclic compounds and there are at least three volume on fused pyrimidines. I am pretty
sure these books will cover what you want. Try volume 24 part II, page 428 onwards, this looks promising. If you have trouble getting it U2U me.
