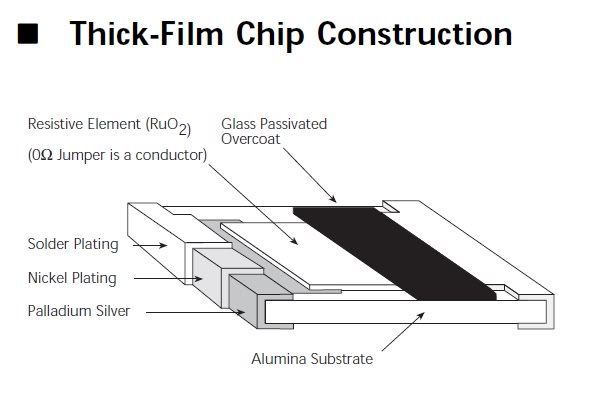
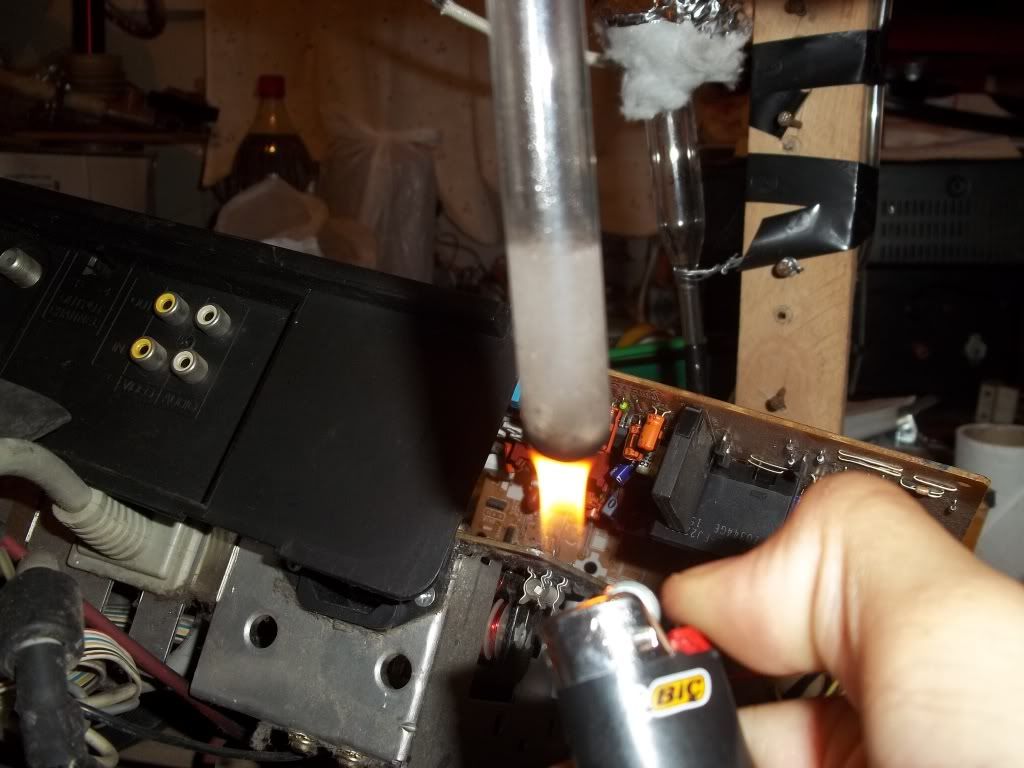
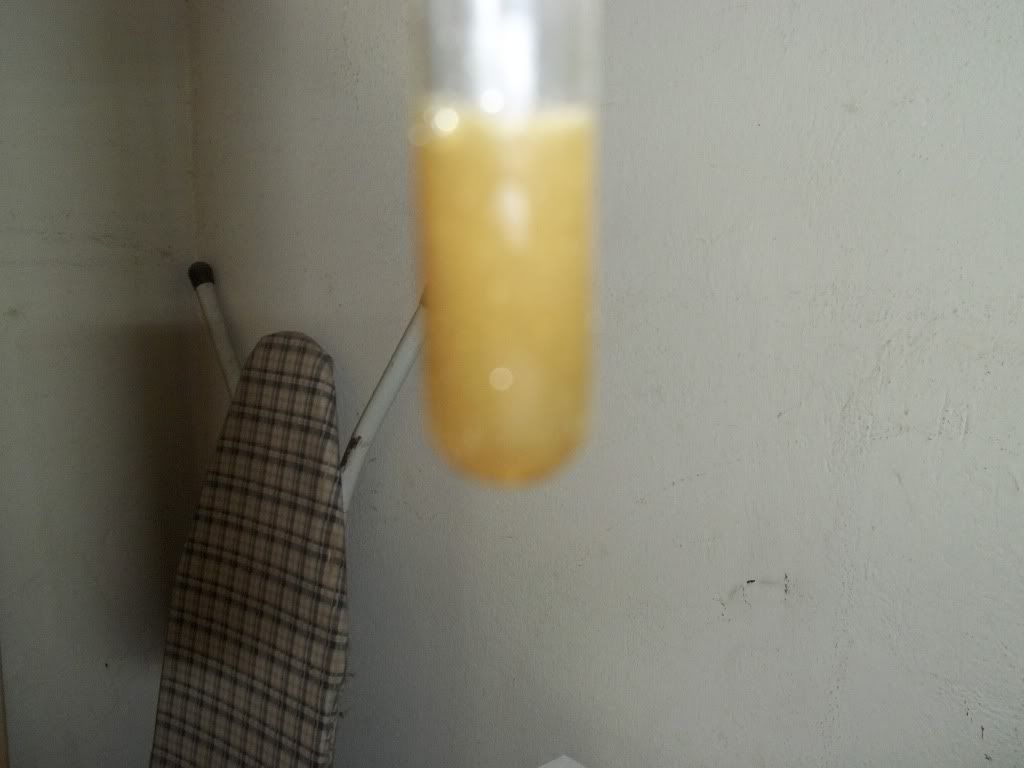
Okay so to recap the procedure described above:
1) remove the chip capacitors and resistors from the board...
2) crush them with a hammer to make a coarse powder (The dust is very harmful, work with dust mask)
3) put the powder in conc. HCl and heat the mixture until small purple-ish crystals form.
4) Add water and separate the soluble metal chlorides from the insoluble remaining debris
by decanting the solution from the solids.
The solution should contain mostly the soluble chlorides of Tin, Iron, Nickel, Barium, traces of Lead...
The solids may contain Palladium, Platinum and some Silver and Lead Chlorides
5) put the solids in HCl and add some Nitric Acid to produce Aqua Regia. Heat until all solids dissolve and produce an orange solution.
6) Test it with Tin Chloride for presence of Pt and Pd.
7) adding HCl to this solution should precipitate the silver first, leaving the Pt and Pd in solution. (The solution is
already chloride based, more HCl will not change the solubility of Ag/Pb-Cl, adding some ice cube or ice bath or 3x times dilution is needed to remove
Ag/Pb-Cl from solution)
8) Add metallic zinc, which will displace the Pd by precipitating it. (Zinc will displace every metal beneath it on the
reactivity series, the black precipitate are subjected to following seperation and refining)
9) The final solution should contain Zinc and Platinum. (irelevant)
10) Test it with Tin Chloride for presence of Pt. (irelevant)
Is my account accurate, so far?
I'd be interested to try this during the weekend...
Robert
|


 ) but the only thing I found was a mention
of densest here: http://www.sciencemadness.org/talk/viewthread.php?tid=13225#...
) but the only thing I found was a mention
of densest here: http://www.sciencemadness.org/talk/viewthread.php?tid=13225#... 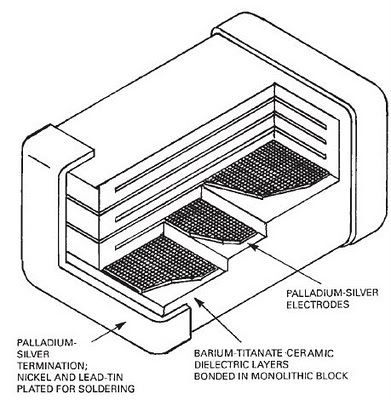

 ). To remove it, just grab one of it and give a little torque, lift
and drop in a small bottle.
). To remove it, just grab one of it and give a little torque, lift
and drop in a small bottle.


 ):
):
 )?
)?