
Ramiel - 6-10-2002 at 00:36
Just to alleviate the pressure on the DPPP subject in the "energetic materials" section i decided to ask about the manufacture of Phorone here.
So! any useful theories on either isolating phorone or producing insoluble phorone?
madscientist - 6-10-2002 at 06:43
Here's my idea.
Heat HCl and CH3COCH3 together until the solution turns yellow. Now add a stoichemtric amount of NaOH to neutralize the HCl. I'm
thinking that the HCl is causing the usually water-insoluble phorone to become soluble.
rikkitikkitavi - 6-10-2002 at 09:37
madscientist, by heating you mean boiling under reflux?
/rickard
BrAiNFeVeR - 7-10-2002 at 08:33
I tried madscientists idea, and it appears to work, I heated for 2 hours, let it cool a bit (it was pretty deep yellow, slowly going to orange)
Then I added some NaOH (didn't measure) and a yell ppt formed (kinda fluffy) which was about half the volume when in solution.
Then I filtered, but while in the filter under liquid, it redissolved somehow !?!
And left me empty handed :-(
Could someone try this again ?
madscientist - 7-10-2002 at 12:48
What was the pH of the mix after adding the alkali?
I wouldn't be surprised if the double bonds present in phorone is responsible for the dissolving. An example of what I'm referring to:
CH2=CH2 reacts with HCl to form CH3CH2Cl.
Brainfever's experiment may have resulted in a nucleophilic substitution with OH-.
Perhaps we should re-evaluate the idea of preparing phorone with an electrophilic catalyst such as HCl. I really don't have time to think this out
right now, but I'll definitely look at it more closely later (this just occurred to me, I've only had a few minutes to consider it).
I doubt it
PrimoPyro - 8-10-2002 at 03:25
It sounds to me like what happened was you polymerized your acetone with the HCl, and then depolymerized it with base back into acetone.
Chloride ion attacks the positive carbon atom of the carbonyl group, and the proton goes to the oxygen, forming the (CH3)2C(OH)Cl intermediate, which
reacts with another molecule of acetone, or another molecule of this intermediate, to form a polymer, either straight chain or cyclic, I am unsure.
Addition of alkalai depolymrized the gem polyether back into the ketone.
PrimoPyro
Hm I wonder
PrimoPyro - 8-10-2002 at 03:30
I wonder if concentrated solutions of HCl and KHSO4 could cause the following reaction, which is highly interesting:
Addition of -Cl to the carbonyl, and H+ to the oxygen in acetone, forming (CH3)2C(OH)Cl
Then the dehydratory strength of KHSO4 causes elimination of water from the halohydrin:
(CH3)2C(OH)Cl = CH3C(CH3)(OH)(Cl)
CH3C(CH3)(OH)(Cl) ==dehydration==> CH2=C(Cl)-CH3
This is 2-chloropropene of course, which could be very useful (to me) in abstract organic synthesis. Interesting.... I wonder?
PrimoPyro
Damn man.....
PrimoPyro - 9-10-2002 at 04:03
WTF is up lately??
These threads all seem so active, and then I make a post and they die instantly.
You guys dont have anything else to talk about now? Damn.....
PrimoPyro
Messes you can make with acetone
Polverone - 9-10-2002 at 11:02
I have treated acetone with HCl until it turned a deep red-brown, then neutralized it with sodium carbonate solution. I obtained a tiny amount of
fluffy yellow precipitate that remained stable over several days, as well as beads of a yellowish oil floating on the surface and a light yellow
aqueous phase.
I have treated acetone with NaOH pellets until it turned a deep-red brown, then added water. The water became a deep reddish color, but a phase formed
above it that was immiscible and even darker. When I added enough citric acid to make the solution slightly acidic (pH 5 or so), the bottom aqueous
phase turned clear yellow and the top phase lightened a bit but remained reddish-brown and immiscible. Make of this what you will.
In both cases, the liquid had a scent somewhat like that of acetone, but it was definitely changed too. There was sort of a sweet sugary smell to it.
I am sure there remains a complex mixture of "stuff."
Ramiel - 9-10-2002 at 20:15
I suggest adding HCl while the whole solution is at 0C and heating it to 20C over a few hours, but not before it turns orange or red (which i think
must be devastation of the phorone). Then neutralize with a bi-carbonate. This seems to be the most likely procedure, any thoughts?
I wonder if
Boob Raider - 15-10-2002 at 09:43
not all the acetone polymerizes to phorone and the phorone that is formed forms that yellow-green soln with the unreacted acetone. So once the
desired color is reached, CaCO3 is added to kill the acid and then the soln is heated to say 60*C to boil off the Acetone. This should leave an oil
(phorone) which should freeze below 28*C into yellow-green prisms.
Hmmm
Polverone - 15-10-2002 at 16:14
That sounds like a fairly reasonable idea. CaCO3 shouldn't affect anything but the HCl due to low solubility. I wonder how much phorone is actually
produced by this method. I have found relatively little reference information on phorone, its properties, uses, and preparation.
What if
Boob Raider - 15-10-2002 at 19:21
I was thinking that if the Cl- is crucial for the formation of Phorone from Acetone then an X amount of conc. H2SO4 + HCl should benifit the reaction
as for every 3 moles of Acetone 2 moles of H2O are formed and 1 mole of Phorone.
Hoffmann-LaRoche - 16-10-2002 at 16:39
exactly my thought too....but i dont know which hydrate of H2SO4 to use would be best, cause concentrated H2SO4 would be too strong and would lead to
carbon, also the H2SO4 itself would react with acetone, i guess.
There is also the danger that mesitylene forms.
Maybe instead a try with very dilute acid, H2SO4*4H2O or H2SO4*5H2O, for example?
We could also try anhydrous CaSO4 to bind the water.
This would result in CaSO4*2H2O, a thick sludge which contains an acidic solution of phorone and the byproducts.
The sludge would then be washed with a strong, not water scavenging acid to get a (very) dilute solution of phorone.
This solution could then be made neutral with CaCO3(note: dont use solutions of NaHSO4 or dilute H2SO4, as they are cheap, sure, but will form CaSO4
which would adsorb all the precious phorone!!)
to get the desired phorone containing product.
HLR
I wonder...
TheBear - 17-10-2002 at 23:19
Couldn't one lead HCl (g) through warm acetone (30*C?) in small amounts during a couple of hours/days until the HCl (g) just bubbles through it and
leaves (I thought that this might indicate that the reactions finished). Then you take your yellow oil and let it cool to 0*C? and if not the whole
mass becomes solid chrystals should form. If not even chrystals forms you may try to pour the liquid into a large (a couple of litres) bowl of
icecold-water to get the, in water insoulabe, phorone.
It's just a thought, please correct my misstakes. 
Polverone - 18-10-2002 at 08:39
The liquid will most likely retain the HCl, leading to unwanted higher condensation products. The HCl catalyzes this reaction, so you can't just wait
until it looks like the acetone has used up all the HCl you're bubbling.
Ramiel - 19-10-2002 at 05:09
Exuse my ignorance, but is HCl(g) soluble in acetone or acetone-like substances?
I suppose so; but just to be sure...
madscientist - 19-10-2002 at 09:05
HCl should be soluble in acetone.
Hoffmann-LaRoche - 19-10-2002 at 10:31
.....and it is also soluble in diethyl ether(Et2O), if that is of interest too....
HLR
Aaron-V2.0 - 19-10-2002 at 14:09
On E&W nbk mentions passing gaseous Hcl through the Acetone. Would this be as simple as placing Hcl into a flask, capping it with a glass tube
leading into the bottom of a beaker full of Acetone, then applying heat to the Hcl?
Hoffmann-LaRoche - 20-10-2002 at 03:46
http://www.sciencemadness.org/talk/viewthread.php?tid=179&pa...
Self-condensation of acetone
BASF - 8-11-2002 at 10:14
Text from
http://www.library.uu.nl/digiarchief/dip/diss/1977553/c1.pdf
The Self-Condensation of Acetone
The self-condensation of acetone towards diacetone alcohol (DAA), shown in Figure 2, is
a typical example of this type of reaction and has been extensively studied.Although relatively
simple on first sight, catalytic self-condensation of acetone is a very complex reaction. Many
products are possible via competitive self-condensation and cross-condensation reactions, like
mesitylene, (iso-) phorone and several isoxylitones, due to the reactivity of both products and
reactants [7].The conversion of acetone towards DAA is equilibrium-controlled and decreases
with increasing temperature as depicted in Figure 3 [8-10]. For this reason, the temperature
during reaction is usually kept below 293 K to obtain a DAA yield in the range of 10-20 wt%.
12
O O O OH
-H2O
Mesityl oxide
Base
2
DAA
Figure 2: Self-condensation reaction of acetone.
The use of alkali hydroxides like NaOH or KOH as catalysts is widespread and gives rise to
several practical problems. Dehydration yielding mesityl oxide (MO) occurs rapidly under
acidic conditions, which is difficult to prevent during neutralization with diluted acid
solutions. Furthermore, remaining alkali catalyst reduces the amount of DAA during
distillation, since then the temperature is in favour of the reverse reaction towards acetone
(Figure 3).The solubility of alkali catalyst in acetone is low at these mentioned temperatures
and for that reason aliphatic alcohols, e.g., methanol or ethanol, are often added [11]. In earlier
years, benzene addition was applied for the same reason [12]. Table 3 summarizes results,
mainly from patent literature, of liquid-phase DAA production using alkali catalysts.
13
Introduction
0
5
10
15
20
25
270 280 290 300 310 320 330
Temperature (K)
DAA (wt%)
Figure 3: DAA production as function of temperature: F from [8], B from [9], J from [10].
Table 3: DAA production with alkali catalysts in literature.
Reference Catalyst Temperature DAA yield Details
(K) (wt%)
Craven [8] NaOH 253-303 27 Significant triacetone alcohol formation
Hawkins [11] KOH 273-298 17 Series of reactors with decreasing
temperature
Lichtenberger [12] NaOH 273-293 15 Benzene used for dispersion catalyst
Teissier [39] NaOH 273 19
To get around the above-mentioned problems, several heterogeneous systems exemplary
for catalysts in liquid-phase condensations have been investigated. Main advantages are the
ease of separation and the re-usability of the catalysts, but they lack the high activities obtained
with the homogeneous catalysts. For instance, Muzart [13] used 0.11 g basic alumina per
mmole acetone, although this method was not intended for commercial use (Table 4).
However, also in patent literature an acetone/catalyst weight ratio as low as 3.3 was applied
[14]. Zhang et al. found a marked increase in activity with MgO when water was added [15].
Therefore, basic OH- ions, i.e., Br©ªnsted sites either retained on the alkaline earth oxide
surface or formed during dehydration of DAA, are proposed as active sites for aldol addition
of acetone in the liquid phase [15,16].
Other applications of the acetone self-condensation can be found in vapour-phase
reactions, typically around 573 K. Products formed are used for the synthesis of various higher
boiling products [7]. Due to this high temperature, dehydration of DAA to MO readily
occurs. For this reason, DAA is usually not found as a product in vapour-phase reactions.
Reaction of acetone and MO results in formation of isophorone and phorone. Preferred
catalysts are certain metal oxides, because hydroxides undergo (partial) dehydroxylation under
the applied reaction conditions. Di Cosimo et al. studied the vapour-phase aldol condensation
of acetone over MgO promoted with 0.7-1.0 wt% of alkali (Li+, Na+, K+, Cs+) or alkaline
earth (Ca2+, Sr2+, Ba2+) metal ions. The basicity of MgO increased on addition of the
promotor following the basicity order of the metal oxide. Addition of acetic acid to the feed
inhibited the reaction, this in contrast to pyridine, indicating that basic sites catalyze the
acetone self-condensation.The nature of the basic site in MgO at these high temperatures was
14
Chapter 1
Table 4: Liquid-phase DAA production with heterogeneous systems.
Reference Catalyst Temperature (K) DAA yield (wt%) Details
Muzart [13] ¥ã-Al2O3 296 11(1) 20% mesityl oxide
Schlenk [14] Ba(OH)2 331 75(2) 1.2 kg Ba(OH)2
on 4 kg acetone
Zhang [15] Alkaline 273 0-10(3) BaO, SrO, CaO, MgO,
earth oxides La2O3,,SiO2-Al2O3, ZrO2,
Nb2O5
Dabbagh [16] Metal 316 Up to 50(4) Mg, Sr, Ca, Ba, Be, La, Al,
hydroxides Th hydroxides
1) Estimated from acetone conversion
2) A soxhlet-type aparatus was applied
3) Highest levels for MgO and CaO
4) A soxhlet was used.
attributed to low coordination oxygen anions O2-.However, surface basic hydroxyl groups, as
in the liquid phase,were not excluded [17].Walton and Yeomans followed a different approach
by using KOH in a high-pressure column at 523 K.A water/acetone (80/20 w/w) azeotrope
reflux containing a KOH concentration in the range of 300-1000 ppm within the reaction
column was used. In this manner, a selectivity of 86 % towards isophorone was obtained.
However, only low acetone conversions up to 8 % could be achieved at this temperature
from camphor??
BASF - 8-11-2002 at 10:40
Phorone
(Phor"one) n. [Camphor + acetone.] (Chem.) A yellow crystalline substance, having a geraniumlike odor, regarded as a complex derivative of acetone,
and obtained from certain camphor compounds.
Interesting....
rikkitikkitavi - 9-11-2002 at 00:26
I searched for phorne at www.depatis.net and came up with a japanese patent
JP0002962842
on how to synthesise phorone
and tried to search for it at www.jpo.go.jp (japanese patent office webpage) but I do NOT have patience work out how to search that page and come up with a positive search
result..
So anyone with more patience than me, have a go for it.
/somewhat frustrated rikkitikkitavi
phorone-batch with photos
BASF - 13-11-2002 at 15:58
A) A batch consisting of 30mL 30% HCl and 20mL of commercial acetone in a loosely stoppered flask was heated for several hours until the liquid had a
golden yellow colour.
[img]http://link.freepichosting.com/image.cgi/11551/2.jpg?x=600&y=400[/img]
The remaining HCl was neutralized by addition of solid sodium carbonate and i recognized that a yellow-white pptate formed while the liquid cleared
up.
The pptate was filtered off and washed with about 500mL of cold water.
Then i dried the pale yellow to white pptate on a water bath(it was actually steam) and weighed it.
[img]http://link.freepichosting.com/image.cgi/11551/1.jpg?x=600&y=400[/img]
Yield of product was 1,5 g.
The product was insoluble in any of the common organic solvents(etOH, hot H2O, acetone, xylene, petrol...).
Soluble in dilute HCl and dilute H2SO4 forming a deep red solution.
[img]http://link.freepichosting.com/image.cgi/11551/3.jpg?x=600&y=400[/img]
(The sample on the photo was a very dilute one.)
B)The same mixing proportions and volumes with extended reaction time.
(Deep red colour of liquid.)
[img]http://link.freepichosting.com/image.cgi/11551/4.jpg?x=600&y=400[/img]
[img]http://link.freepichosting.com/image.cgi/11551/4.jpg?x=600&y=400[/img]
The above process was repeated and 1,7 g of the product were obtained.
C)The same again.
Waited just more till the liquid was a deep dark red, like a blood conserve.
Yield was again 1,7g.
Funny: the powder seems to be an acceptable pH indicator for pH 7: being dark to light red in acidic media, decolourizing at pH 7.
[img]http://link.freepichosting.com/image.cgi/11551/5.jpg?x=600&y=400[/img]
HLR
BASF - 13-11-2002 at 16:01
what the fuck.....did anyone try to import photos yet?
....u can still paste the urls....sorry
madscientist - 13-11-2002 at 18:07
I think it's clear that the addition of the sodium bicarbonate initiates a more complex chemical change than an acid-base reaction.
madscientist - 13-11-2002 at 18:11
Whoops, I meant to say sodium carbonate.
I forgot to ask: was there fizzling upon addition of the sodium carbonate to the yellowish solution prepared by heating acetone and HCl together?
BASF - 14-11-2002 at 10:58
Yes, there was fizzling.....it was (as expected) a very vigorous reaction.
The addition had to be done very slowly.
I did NOT add an excess(stopped the addition at ph7-8) and immediately washed the precipitate with large amounts of water.
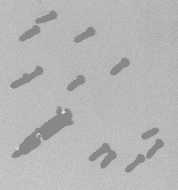
Krypton - 27-11-2002 at 12:31
I`ve found a pic of phorone crystals in my archive. 
Haggis - 13-8-2003 at 13:36
This is an older thread..sorry for dredging it up. Recently, I attempted Phorone with 33.25 ml HCl and 42.73 ml Acetone. I did my mixing with room
temperature chemicals. I added the acid fairly rapidly, in around 4 steps, whilst stirring vigorously. After the addition of the acid, the temperature
was quite high. For some reason, I decided to cool it down. Before placing in the freezer, it was slightly foggy, but otherwise colorless water. It
was placed in a -5 degree celcius freezer for one hour. After one hour, the liquid was clear, losing the bit of fogginess within. I set the flask in
room temperature darkness and promptly forgot about it. About 18 hours later, the liquid within the flask has turned a yellow a bit darker than urine.
I let it set back. 6 hours later, the solution is barely darker and with a slight tinge of orange. I tried to add a bit of sodium carbonate. I
expected to see fizzing and bubbles as the acid is neutralized. The bit of crystals simply sunk to the bottom. After much swirling, only a slight bit
of the mass on the bottom has dissolved, and the amount put in was the ever scientific 'half of a pinch'. The sodium carbonate isn't
forming any bubbles at all. Also, the solution smells similar to acetone, with a strange twang to it. I am letting it set some more, seeing if it will
turn to a darker red.
The_Davster - 18-7-2004 at 23:13
I am bringing this thread up from the depths of at least the sixth page in the organic chemistry section, I hope thats okay.
I found this reaction in "Textbook Of organic chemistry" Noller, 1958.
"Under more vigorous conditions of catalysis and dehydration, higher condensation products are formed
3CH3COCH3--->phorone+ 2H20"
The conditions that were above the arrow are dry HCl, ZnCl2 or AlCl3.
Since dry HCl is required I assume that the acetone should be dry as well.
Also, since "vigorous... dehydration" was stated, it may be necessary to remove the water formed in the reaction. However, since
concentrated sulfuric acid will not work for drying out the reaction in this case(production of mesitylene), a more suitable drying agent must be
found. Ideas?
I would try this but my acetone is not anhydrous.
Esplosivo - 19-7-2004 at 02:53
As a drying agent I think anhydrous Sodium sulphate could be used. It is highly unreactive, and I suppose slightly soluble in anhydrous acetone.
Edit: Btw, anhydrous Na2SO4 can also be used to remove the water in the azeotropic acetone/water mixture.
[Edited on 19-7-2004 by Esplosivo]
Marvin - 19-7-2004 at 13:23
An old method of producing metisyl oxide and phorone Ive mentioend before. Saturate acetone with dry HCl and leave for a day or two until it aquires
a yellow colour. Neutralise with alcoholic potassium carbonate. Adding water to the product should produce an insoluable oil which is a mixture of
phorone and metisyl oxide. These are seperated by distillation.
Replacing the neutralising agent with CaCO3 seems like a good move as mentioned earlier.
Having said this, it isnt clear if phorone peroxide can actually be made from phorone. I'm not aware double bonds usually peroxide easily with
hydrogen peroxide.
new route to phorone?
Intergalactic_Captain - 27-12-2004 at 19:12
First off, I will not pretend to know the chemistry behind an Aldol condensation reaction. I will, however, share my thoughts and observations on the
matter. I'm sorry for digging up an old thread, but it's probably better than starting a new one.
Now, lets put on our thinking caps. Acetone and H2SO4 condense primarily to mesitylene, along with some other aromatic and possibly straight chain
crap. HCl will condense acetone to phorone. Or dichlorophorone, or pentachlorophorone...depends who you ask. Either way, you get A phorone from
HCl. BaOH supposedly consenses acetone to Mesityl Oxide.
Many acids and bases have been seen to provide different condensation products. I believe, though, that good old lye may be the answer. Yesterday, I
put a small jar of aqueous NaOH (unsure of concentration, but probably around 20% by weight) and acetone, equal volumes, on a hotplate at around 65 C
for roughtly 6 hours. Over time, the upper acetone layer took on a pale yellow color. Unfortunately, the lye phase and acetone phase were
immiscible, leaving a very small reaction zone.
Today, I separated and evaporated the acetone phase. I was left with a few grams of a non-crystaline yellow mass. It was definitely a lachrymator,
but did not smell of acetone. By its physical properties, I have ruled out the possibility of it being Mesitylene, Mesityl Oxide, Isophorone, and
Diacetone Alcohol. Unfortunately, I still can't figure out exactly what I have. If anyone could field a more educated guess than mine, it would
be greatly appreciated.
Currently, I have a jar of 300ml of each of ~25% NaOH soln. and acetone, with the acetone floating atop the lye. Over the past 2 hours, at room
temp., the acetone has taken on a very light yellow hue, suspiciously charectaristic of the acetone coloration described in the HCL/acetone procedure
for phorone. I plan to leave this overnight, and will update everyone on its progress sometime tomorrow.
Intergalactic_Captain - 29-12-2004 at 10:14
My above mentioned lye/acetone mix has now been reacting for about 36 hours at room temperature. The acetone layer has taken a bright, translucent
yellow hue, while the lye layer is completely clear. If anyone is interested, I will post a picture...I just need to install my digicam software on
my laptop, but I'm a lazy lazy man.
I plan to let the mix react until no further color change is seen. I've been shaking it a few times a day to achieve an emulsion, but the
emulsion breaks quickly. If anyone knows of an inert emulsifier that I could use to keep the mix emulsified and hopefully speed up the reaction, I
would be greatly appreciative.
guy - 25-8-2006 at 11:03
I made some phorone with solid NaOH and acetone and heat it up (not boiling) overnight. All was left was a black solid-goo (contains NaOH and
phorone). The mixture smelled strongly of some kind of wood (I presume that it is camphor-like).
Dissolve this in as little acetone as possible.
Then dilute it with water until phorone precipitates. There should be a red goo floating on top. This red good is responsible for the red black
color and can be easily removed with a toothpick.
Now evaporate the water and redissolve in some acetone to remove NaOH. (Solution should be a bright yellow color only)
Evaporate the acetone to have almost pure phorone.
[Edited on 8/25/2006 by guy]
[Edited on 8/25/2006 by guy]
Nicodem - 27-8-2006 at 23:12
What makes you believe that what you obtained is phorone? What kind of analysis did you perform?
Somehow I doub't you can evaporate a water solution of phorone (for that little that is soluble) without steam disstiling off any potential phorone.
Anyway, NaOH and acetone gives diacetone alcohol (4-Hydroxy-4-methyl-2-pentanone).
guy - 29-8-2006 at 20:48
I don't know for sure but it smells like phorone and looks like phorone. Phorone is slightly soluble in water and has a much higher boiling point
than water (around 200). NaOH and acetone does give diacetone alcohol, which will dehydrate to form mesityl oxide, isophorone, phorone, and some
other stuff. Phorone is the only solid at room temperature.



