<table><tr><td><div align="center">13. GAS GENERATORS</div>
(a) <em>Carbon Dioxide, Hydrogen, an Hydrogen Sulphide.</em> The simplest form of generator for these gases is shown in Fig. 7. The
solid material, cracked marble for carbon dioxide, feathered zinc for hydrogen, and ferrous sulphide for hydrogen sulphide is placed in the 300-cc.
thick-walled generator bottle. The tubes are fitted as shown, and in the drying tube is placed a plug of cotton wool to strain the acid spray out of
the gas, or if the gas is to be dried, granulated calcium chloride held in place with a plug of cotton wool on either side. Enough water is poured in
through the thistle tube to cover its lower end and then about 5 cc. of 6 <em>N</em> HCl. The gas begins to generate rather slowly, but
if one is impatient and adds more acid at once the reaction will soon become so violent as to drive foam out through the delivery tube. After a few
minutes add more acid, 1 cc. at a time, in order to keep up the evolution of gas at the desired rate.<br /><br />–
<em>Synthetic Inorganic Chemistry: a Course of Laboratory and Classroom Study for First Year College Students</em>. Blanchard, Phelan,
& Davis. 5th ed., Wiley & Sons, 1936.</td><td>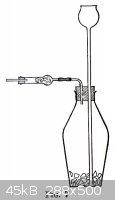 </td></tr></table> </td></tr></table> |













 .
.











 .
.
