| Question 2: I noticed that the yellow layer, supposedly containing most of the benzyl alcohol, dissolved very well in blue spirits (which turned a bit
greenish). Is it worth trying to add H2SO4 to an ethanolic solution of the hardener, to separate out the sulfate of isophorone diamine? Is it okay to
use ketonated alcohol as the solvent, instead of pure alcohol (compatibility wigh reaction)? |


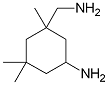
 is likely to be more soluble in
water.
is likely to be more soluble in
water.


