


Quote: Originally posted by Mailinmypocket  |


Quote: Originally posted by kristofvagyok  |
Quote: Originally posted by elementcollector1  |



Quote: Originally posted by Arthur Dent  |




Quote: Originally posted by kristofvagyok  |
Quote: Originally posted by Hegi  |
Quote: Originally posted by kristofvagyok  |


Quote: Originally posted by bfesser  |

 never seen large phenol crystals in person
never seen large phenol crystals in person



Quote: Originally posted by Bot0nist  |
Quote: Originally posted by Magpie  |
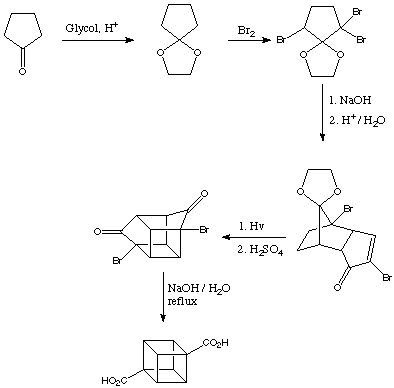

Quote: Originally posted by Bot0nist  |
Quote: Originally posted by Hegi  |
Quote: Originally posted by ElizabethGreene  |

Quote: Originally posted by bfesser  |
 This was to produce ~100ml.
This was to produce ~100ml.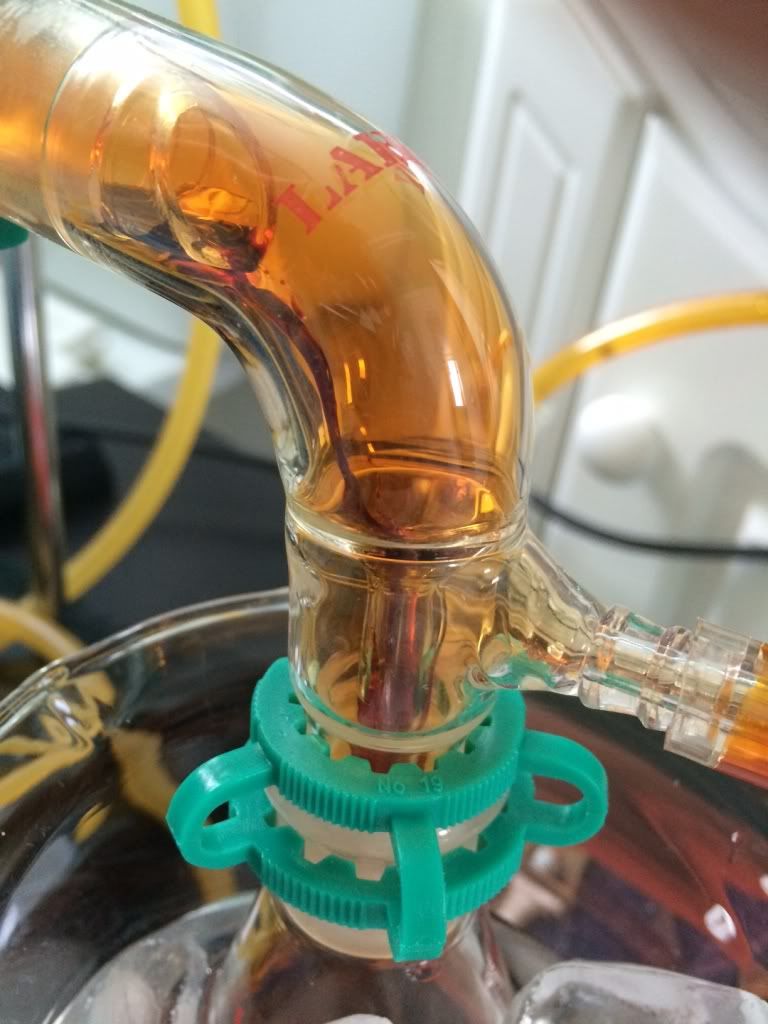 [/img]
[/img]
 . It was a lot of fun/learning/challenge/frustration through many months, it is not really hard but I started doing
this 5 months ago when I just started chemistry as a hobby and I also had to wait to get more chemicals and labware to make different platings. The
next one will be silver when I'll finaly manage to get HNO3 to dissolve it, Nitrates are so hard to get in Canada!
. It was a lot of fun/learning/challenge/frustration through many months, it is not really hard but I started doing
this 5 months ago when I just started chemistry as a hobby and I also had to wait to get more chemicals and labware to make different platings. The
next one will be silver when I'll finaly manage to get HNO3 to dissolve it, Nitrates are so hard to get in Canada!Quote: Originally posted by Brain&Force  |



 Anyways, I took some pictures of burning zinc
after that. The quality kind of sucks, but it is still acceptable.
Anyways, I took some pictures of burning zinc
after that. The quality kind of sucks, but it is still acceptable. 
 I
used our maple syrup as a source of glucose as I didn't manage to find pure glucose but it's even better ! It really looks like beer :p
I
used our maple syrup as a source of glucose as I didn't manage to find pure glucose but it's even better ! It really looks like beer :pQuote: Originally posted by Brain&Force  |
 soon... Now it´s being recrystallised..
soon... Now it´s being recrystallised.. Quote: Originally posted by Hegi  |


Quote: Originally posted by bfesser  |
Quote: Originally posted by kristofvagyok  |


Quote: Originally posted by Brain&Force  |

Quote: Originally posted by Oscilllator  |

Quote: Originally posted by Zyklonb  |



Quote: Originally posted by MrHomeScientist  |