

Quote: Originally posted by DraconicAcid  |



Quote: Originally posted by Zyklonb  |
 Probably not.
Probably not.


Quote: Originally posted by MrHomeScientist  |


Quote: Originally posted by violet sin  |
 There is such
a low barrier to entry for electrochemisty and a vast number of experiments to be performed, I wish I saw more of it on this forum.
There is such
a low barrier to entry for electrochemisty and a vast number of experiments to be performed, I wish I saw more of it on this forum.Quote: Originally posted by Töilet Plünger  |


 is what I should have said. I plan on making a nicer one now that I have a better camera and apps for this type of filming. I have a few indicator
mixes if like to try as well including a fluorescent one, so, we shall see how that works out
is what I should have said. I plan on making a nicer one now that I have a better camera and apps for this type of filming. I have a few indicator
mixes if like to try as well including a fluorescent one, so, we shall see how that works out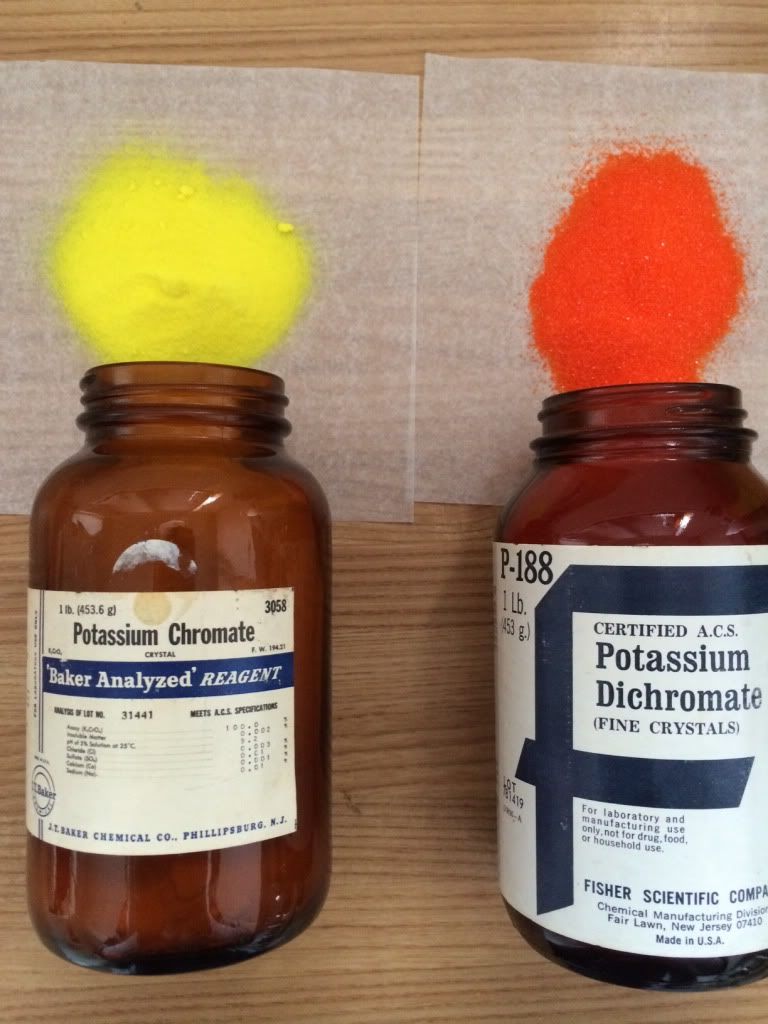

Quote: Originally posted by Mailinmypocket  |
 i see alot of the pics here are of some type of crystalline formations. the
silver metal on the copper wire look pretty neat. i tried the same but could not get decent close up pics.
i see alot of the pics here are of some type of crystalline formations. the
silver metal on the copper wire look pretty neat. i tried the same but could not get decent close up pics.Quote: Originally posted by NeonPulse  |



Quote: Originally posted by Brain&Force  |

Quote: Originally posted by Hegi  |




Quote: Originally posted by Oscilllator  |
Quote: Originally posted by numos  |
Quote: Originally posted by BlackDragon2712  |
Quote: Originally posted by HeYBrO  |

Quote: Originally posted by Hegi  |







Quote: Originally posted by Brain&Force  |





Quote: Originally posted by Oscilllator  |
 Anyone tried mutating bacterium who have the gene? Would
be interesting... I don't have anything for such a procedure, but would love to see someone try! Maybe give them some light carcinogen...? If such a
thing exists
Anyone tried mutating bacterium who have the gene? Would
be interesting... I don't have anything for such a procedure, but would love to see someone try! Maybe give them some light carcinogen...? If such a
thing exists 
Quote: Originally posted by DraconicAcid  |


 .
.


 Any flowers may not take copper sulfate
(which happens to be sold as root kill) too well!
Any flowers may not take copper sulfate
(which happens to be sold as root kill) too well!Quote: Originally posted by Pyro  |
Quote: Originally posted by The Volatile Chemist  |
Quote: Originally posted by Bert  |
Quote: Originally posted by Bert  |
Quote: Originally posted by Brain&Force  |








Quote: Originally posted by arkoma  |



Quote: Originally posted by arkoma  |

Quote: Originally posted by The Volatile Chemist  |

