Time to post this - yet again.....
The Wrong Chemical -- In The Wrong Place!!
Donald J Haarmann is The WiZ
Originally published in The American Fireworks News
Potassium dichromate K2Cr2O7 (potassium bichromate, red potassium
chromate). Familiar to most pyros because of its bright orange-red color. Has
many uses:
"Dichromate" magnesium against chemical attack.
Added to compositions containing highly reactive zinc, e.g.,
Weingarts Electric Spreader Star
Potassium dichromate 15 parts
Zinc dust 72
Potassium chlorate 15
Granulate charcoal 12
Dextrin 2
Increase burning rate reaction of: Perchlorates:Re: Dichromate....safety concerns over-rated??
From: "donald haarmann" <donald-haarmann@xxxxxxxxxxxxxxxx>
Date: Tue, 06 Feb 2007 22:26:15 GMT
"mikes2653" <mikes2653@xxxxxxxxxx>|
[snip]
| Still, it is remarkable how much exposure to many toxic substances is
| necessary before even minor symptoms are noted. The healthy human body
| is much more robust than many people believe. Excessive worry about
| environmental toxicity seems to me a species of hypochondria.
Dave Blesers Red Strobe
Ammonium percolate 50%
Potassium dichromate +5
Magnesium 25
Strontium sulphate 25
Shimizus' Burst Charge #44
Potassium dichromate 5%
Potassium perchlorate 70
Hemp coal 30
Rice starch 2+
Takeo Shimizu reports the burning rate of a 75/25 K perchlorate/hemp coal
increased by 55% when 4-5% potassium dichromate was added.
In Orange (toxic) smoke (Ellern #133)
Potassium dichromate 35%
Magnesium 15
Lead dioxide 50
It is not, however, used as an oxidizing agent despite an attracting 38% oxygen
content. he decomposition reaction as given by Takeo Shimizu shows why-
4 K2Cr2O7 --> 4 K2CrO4 + 2 Cr2O3 + 3 O2
A little quick math reveals that - 1176 grams of potassium dichromate yield a
miserly 48 grams of oxygen! (4% of starting weight!) Thus potassium dichromate
is a very weak pyrotechnic) oxidizer. Shimizu also notes; It is difficult to ignite or
to explode a mixture of potassium dichromate and red phosphors or sulphur even
by impact between iron surfaces.
Sadly the pleasing orange color hides a less then pleasant personality. While
classified by he NFPA a Zero for Fire hazard and Reactivity (Will not burn. -
Normally stable. Not reactive with water). It rates a Three for Health (Can cause
serious injury despite medical treatment.) Quote the Merk Index: Human Toxicity:
Internal a corrosive poison. Industrial contact may result in ulceration of hands,
destruction of mucous membranes and perforation of nasal septum. Not a
ingredient a pyro would handled in a careless manner! Non pyros are another
matter.
Civilian uses are as many as they are pedestrian: Tanning, dyeing, painting,
decorating porcelain, photolithograpy, staining wood, bleaching palm oil, wax,
and sponges, in dry cells as a depolarizer.
Then there is South Africa -- Where a third to two-thirds of blacks have been
estimate to receive frequent purgative enemas consisting of plant extracts though
truncated cow horn and hollow reeds administered by traditional healers. These
methods with increasing urbanization are being replaced by domestic and
industrial chemicals given with rubber tubing and syringe. Indeed among the
black population in Cape Town, tribal healers have been using potassium
dichromate in purgative enemas! A bad case of the wrong chemical in the wrong
place!! Even infants may receive 50 enemas a year.(The herbal version, not the
hexavalent chromium-tissue binding, don't try this at home kids, colon/kidney
destroying, dichromate version.)
All this probable proves something. I'll leave it to the reader to supply their own
comments.
Dunnn LP, et al. Colonic complications after toxic tribal enemas. British Journal
of Surgery, 1991; 78: 545-48. Abstracted in The Lancet July 20, 1991
--
donald j haarmann
------------------------------------
What passes for woman's intuition is
often nothing more than man's transparency.
George Jean Nathan
|












 ) can not dissolve bicycle tire inner tube
) can not dissolve bicycle tire inner tube 















 or to make a little show.
or to make a little show.






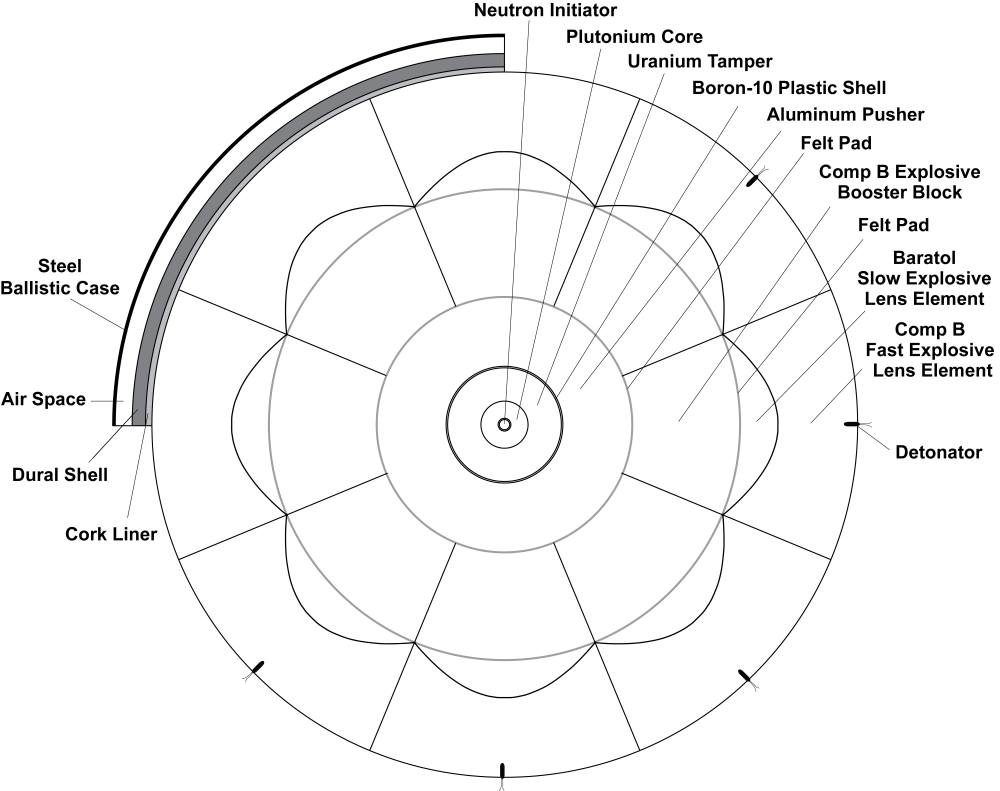
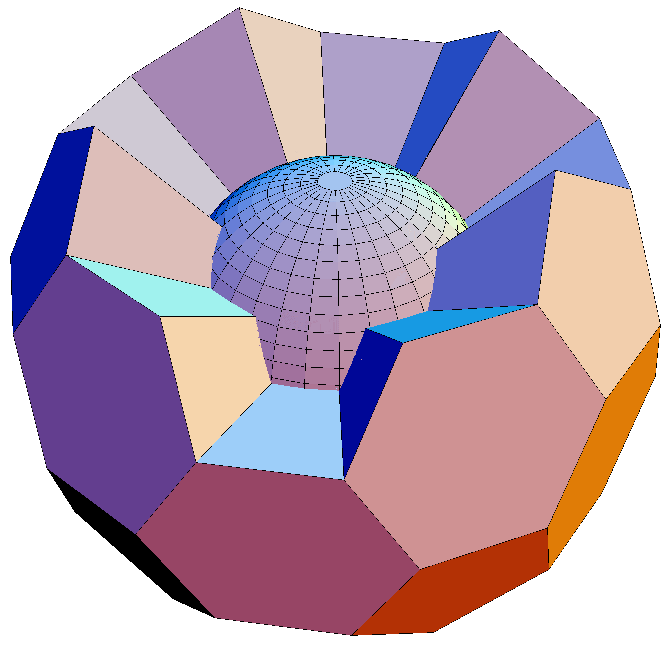









 Yay! Documentation!! Referances AND pertinent quotes from same!!! Patent
information!!!!
Yay! Documentation!! Referances AND pertinent quotes from same!!! Patent
information!!!! 
 Not like some of the others, who make tiny plastic baby Jesus cry, and kill many
kittens.
Not like some of the others, who make tiny plastic baby Jesus cry, and kill many
kittens.













