
tom haggen - 27-1-2005 at 21:08
Anyone have any good tips on how to make electron config. seem easy.
Lattice enthalpy is proving to be a pain in the ass all so. Mid terms are sucking ass this week
Mumbles - 28-1-2005 at 17:12
Perhaps you should share exactly what you are having difficulties with. I know it's never been my best subject. I always have problems with the
d level orbitals where electrons are borrowed and such. As much as I hate to say this, and even much less do it, the best way is lots of practice.
Just get yourself a nice big list of elements and compounds and start practicing. I'm sure there are tests with answers on the internet.
tom haggen - 28-1-2005 at 18:42
Well I guess i'm not having that much trouble. My chem test went a hell of a lot better than my math test. The only part I screwed up on was this
diagram that you fill up with electrons. You had to identify the orbitals as being molecular or atomic or something also had pi and sigma symbols all
over the place. I like lewis structures though, I think i will like o chem better.
[Edited on 29-1-2005 by tom haggen]
Magpie - 28-1-2005 at 18:57
Here's a little trick for the aufbau principle of electron filling:
Draw this first:
7s 7p
6s 6p 6d
5s 5p 5d 5f
4s 4p 4d 4f
3s 3p 3d
2s 2p
1s
Now draw arrows diagonally, up and to the left, through 1s, 2s, 2p, 3p, 3d, 4f, and 5f. This will give you the filling order. Hope this helps.
tom haggen - 28-1-2005 at 19:08
No this diagram was way different than the regular one. It was shaped like a club and had sigma and pi symbols between the orbitals. I cant remember
what kind of diagram it was called. I through away the hand out
Synopsis - 28-1-2005 at 21:41
Are you speaking of atomic orbitals combining to form sigma and pi orbitals?
Like two 1s atomic hydrogen orbitals combining to form a sigma bonding molecular orbital and a sigma antibonding molecular orbital?
You need to be more precise if you wish a concise answer...
[Edited on 29-1-2005 by Synopsis]
mick - 29-1-2005 at 09:37
Are you on about orbital hybridization.
For carbon the 2s 2e and 2p 2e hybridize to give a tetrahedron for CX4, but double bonded carbon is a 2s 2e and a 2p 1e hybridization with a 2p pi
bond which is planar and the carbon triple bond is 2 x 2p pi bonds with the s bonds and is linear, as in acetylene.
or something like that
Explains why water is v-shaped, ammonia is pyrimidal etc
They might have change the ideas or presentation but that's what I learnt and works for me.
The d and f orbitals were always a problem.
mick
[Edited on 29-1-2005 by mick]
tom haggen - 29-1-2005 at 19:56
I got a picture of the diagram that was on my test if you guys are interested. I almost had it filled out correctly but screwed up at the last minute.
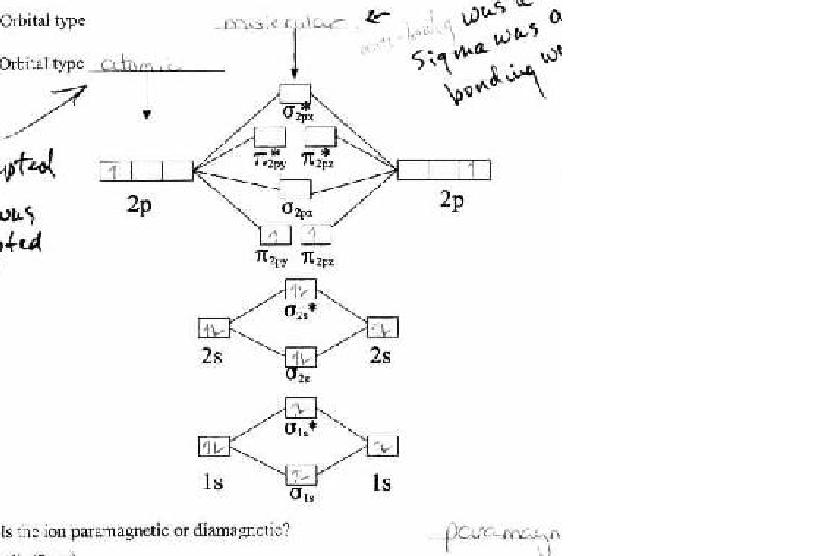
Mumbles - 30-1-2005 at 18:15
Oh those, I don't remember the name of them. I just always called them anti-bonding diagrams. I really have no recolection how to use them.
The sides are for electrons each atom in the bond has, and you fill in the total number of electrons in the middle going from the bottom to the top.
The more electrons that are in the top set of boxes mean a weaker bond. The top set are called teh antibonding orbitals.
More information can be had by searching for "Molecular Orbital Theory". I only remember little bits and pieces so a googling or a text
book reading should really help.


