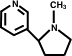
Nicotine, C10H14N2. The pure alkaloid is a colourless oil, b.p. 246.1 °/
730-5 mm. It can be purified
through the crystalline zincichloride, B,ZnCl2,2HCl,H20, the regenerated
base being distilled under reduced pressure (20-40 mm.) in presence of
nitrogen or hydrogen. It distils unchanged in a current of steam and is
readily soluble in alcohol, ether or light petroleum. The behaviour of
nicotine with water has been studied by several workers.19 It is miscible
in all proportions with water below 60° and above 210°; at intervening
temperatures soluble hydrates are not formed and miscibility is limited.
According to Kelly et al.19 an azeotrope is formed, which contains 2.45 per
cent, of nicotine and boils at 99-6°/760 mm. The salts are readily soluble
in water, do not crystallise easily and are dextrorotatory.
...
The acid d-tartrate,
B . 2H2C4H4O8 . 2H2O, m.p. 88-9° (hydrated), and
the neutral d-tartrate, m.p. 68-5° (hydrated), , both
crystallise from alcohol on addition of ether. The dipicrate,
B . 2C6H2(NO2)3OH, short yellow prisms, m.p. 224°, and the tetrachloriodide,
22 C10H14N2.2(HICl4), orange prisms, m.p. 150° (dec.), are
characteristic. |






 this method I could say from my own experience this may be better than
repeated recrystallisations or destillations. Especially if you need some really pure product. And with repeated destillations you wouldn´t have less
work and you risk losses of products (e.g. in decopmposition processes). Also I think especially for extractions of substances from plants where you
may have lots of unwanted derivatives or just shit which could be difficult to remove and the yields are very poor (only some percents) this may be a
good and effective method
this method I could say from my own experience this may be better than
repeated recrystallisations or destillations. Especially if you need some really pure product. And with repeated destillations you wouldn´t have less
work and you risk losses of products (e.g. in decopmposition processes). Also I think especially for extractions of substances from plants where you
may have lots of unwanted derivatives or just shit which could be difficult to remove and the yields are very poor (only some percents) this may be a
good and effective method

