
Raskolnikov - 15-8-2005 at 00:59
Hello! 
I am an italian newbye (excuse me for my terrible english!  ).
).
I have reflected on the reaction of production of TATP (I not to can to ask at my science professor: He not to aswer at this question  )
)
"My God! My english is very very monstrous! I am completely incomprehensible for myself...  "
"
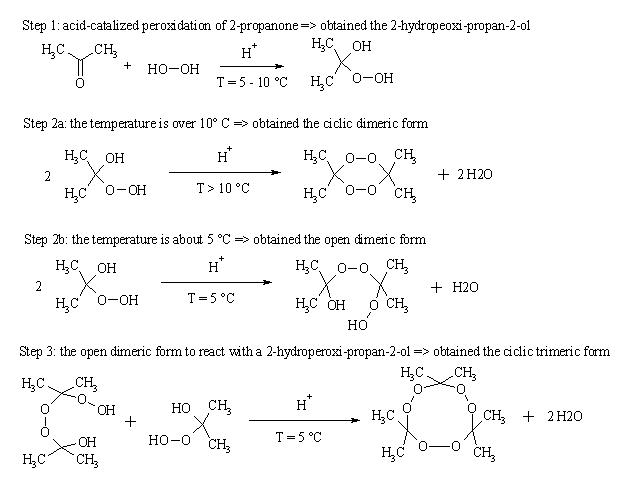
Therefore, I have propose this mechanism (a JPEG image in attachment...)
Is correct?
"Worse and worse... I have to study the english language rather than the chemistry...  "
"
Thanks in advance for your answer! 
Bye!
neutrino - 15-8-2005 at 03:23
This should help.
Raskolnikov - 15-8-2005 at 04:34
I have try already this link... But this page is not available, indeed all the site www.roguesci.org is not available... 
Perharps, this site is sequestrated by the Police? 
Comunque, grazie per l'interessamento...
Anyhow, thank you for interest... 
Bye!
[Edited on 808/1515/05 by Raskolnikov]
chemoleo - 15-8-2005 at 12:26
Yes, I heard that as well that rogue is blocked in Italy. 
Anyway, the mechanism looks good to me at a glance. This is what I remember about it, too.
Dannazione!
Raskolnikov - 15-8-2005 at 13:17
Thank you, chemoleo!
Now the Italian Postal Police to keep an eye on me... 
Good-bye!

solo - 15-8-2005 at 15:53
Raskolnikov....have no fear here is the reference material suggested by chemoleo.......solo
http://rapidshare.de/files/4022224/Megalomania_s_Method_of_M...
Chris The Great - 15-8-2005 at 18:13
Yes, your reaction scheme is correct. I read a journal before, and that is the reaction scheme it described.
EDIT:
It seems that you have the general reaction correct but not the actual conditions. Trimeric acetone peroxide forms the majority of the product when
HCl is the acid, dimeric is the main product when H2SO4 is the acid. Both are of course influenced by temperature, higher temperature shifting the
equilibrium to the dimer.
Also, the H+ serves only to form the cyclic products- non cyclic products form from the acetone and hydrogen peroxide reacting without any acid (they
are water soluble so don't precipitate).
I seem to have rushed through this thread and didn't notice those errors the first time through.
A very good job though 
[Edited on 16-8-2005 by Chris The Great]
Chris The Great - 15-8-2005 at 20:32
Here is a good journal on the reactions in question. As you can see it involves many different equilibriums etc, and is quite a complex reaction
scheme for such a simple procedure.
Attachment: The Reactions of Acetone and Hydrogen Peroxide.pdf (582kB)
This file has been downloaded 940 times
Grazie a tutti!
Raskolnikov - 16-8-2005 at 00:23
Thanks! 
Bye!
Dany - 21-9-2014 at 08:08
The following is a proposed mechanism for the formation of TATP using Raman spectroscopy and DFT calculation. The figure is taken from Ref. [1].
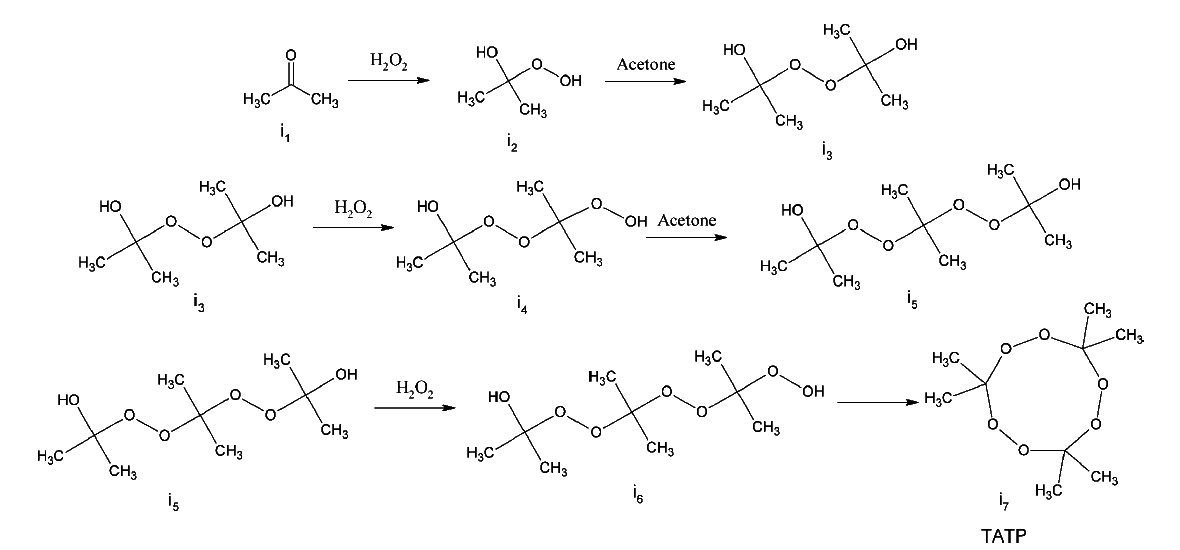
Reference:
[1] Jensen L, Mortensen PM, Trane R, Harris P, Berg RW; Appl Spectrosc., 2009, 63, 1, 92-97.
Dany.
[Edited on 21-9-2014 by Dany]


 ).
). )
) "
" "
"








