


| Quote: |





 ). He told me that I
couldn't as it would be much to expensive, he had to put up an order and all that. The only possible way to go was giving me some spillover. I left
with 3.5 kg of Ti gr1 and MMO-sheet metal
). He told me that I
couldn't as it would be much to expensive, he had to put up an order and all that. The only possible way to go was giving me some spillover. I left
with 3.5 kg of Ti gr1 and MMO-sheet metal

 . WoW!
. WoW!




Quote: Originally posted by Gamal  |



 While you are decanting
and getting ready for a decomp to potassium, I am washing masses of snow-white potassium chlorate crystals. MMO is the King of Chlorate!
While you are decanting
and getting ready for a decomp to potassium, I am washing masses of snow-white potassium chlorate crystals. MMO is the King of Chlorate! of Chlorate Anodes. (pH controll definitely recommended).
of Chlorate Anodes. (pH controll definitely recommended).

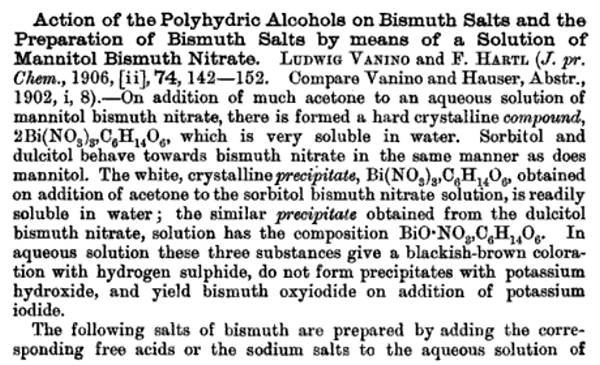
 Take unto thee of
bismuth nitrate two loth and of mannite five, and triturate together well in an agate mortar whilst murmuring the appropriate incantations prior
Take unto thee of
bismuth nitrate two loth and of mannite five, and triturate together well in an agate mortar whilst murmuring the appropriate incantations prior


Quote: Originally posted by tentacles  |
Quote: Originally posted by dann2  |

Quote: Originally posted by Swede  |

 ). It looks like they use teflon
or silikone hoses most.
). It looks like they use teflon
or silikone hoses most.



| Quote: |


 )
)
 is 0.6256 grams. Therefor each gram of
is 0.6256 grams. Therefor each gram of 



| Quote: |
| Quote: |
| Quote: |

Quote: Originally posted by Swede  |

Quote: Originally posted by dann2  |
Quote: Originally posted by Swede  |


Quote: Originally posted by quest  |