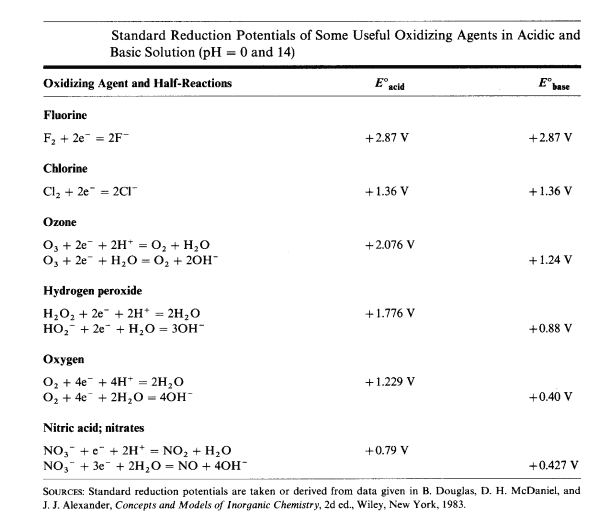
In alkaline conditions, H2O2 oxidises Cr(III) to chromate (CrO<sub>4</sub><sup>2-</sup>, Cr(VI)) easily.
And yet that same Cr(VI), in acid conditions (it is then dichromate, Cr<sub>2</sub>O<sub>7</sub><sup>2-</sup>
 H2O2 reduces Cr(VI) back to Cr(III).
H2O2 reduces Cr(VI) back to Cr(III).What H2O2 does simply depends on pH.
H2O2 + 2 H+ + 2 e === > 2 H2O
2 H2O === > 2 H+ + 2 OH-
---------------------------------------- (sum)
H2O2 + 2 e === > 2 OH-, in alkaline conditions, acts as an oxidiser.
H2O2 === > O2 + 2 H+ + 2 e, in acid conditions, acts as a reducing agent.
It behaves also like that with Ce(III) to Ce(IV) which happens with H2O2 in alkaline conditions. But Ce(IV) is reduced back to Ce(III) by H2O2 in acid conditions.
[Edited on 23-2-2015 by blogfast25]