
Texium - 15-6-2015 at 13:46
The other day when I was making tin(IV) chloride from tin and chlorine, the use of a trap for suck-back of the final NaOH scrubbing solution was
required. I had a 500 mL Erlenmeyer flask as the scrubber, and a 500 mL RBF before it in line to act as a suck-back trap. I used about 400 mL of
scrubing solution. Predictably, there was a lot of suck-back since chlorine is very soluble in NaOH solution. Nearly the entire flask of solution was
sucked back, and because of that, I had to temporarily halt my procedure and disassemble the apparatus so I could refill the scrubber trap. While
doing this, I realized that it would be very nice if the suck-back trap had a stopcock on it that could return the solution to the scrubber when the
trap gets too full, and it prompted me to draw out a plan for this design.
tl;dr I had issues with a conventional suck-back trap and designed a possible improvement.
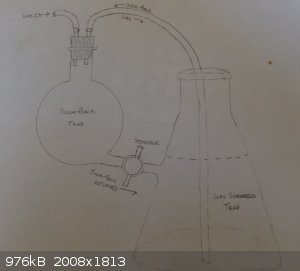
One possible flaw that it might have is that when the suck-back trap is emptied, it might actually cause more suck-back to happen, or won't drain at
all because of creating negative pressure in the trap. I'm not sure though, as I'm really lousy with physics.
Magpie - 15-6-2015 at 14:36
Can you temporarily pull the gas tube up above the scrubber liquid level? If you can do this then open the valve the trap will drain.
[Edited on 15-6-2015 by Magpie]
BromicAcid - 15-6-2015 at 15:25
Usually I just put the outlet of the suck back flask (going to the scrubbing solution) all the way to the bottom of the flask. The inlet is all the
way at the top of the flask. If there is suck back into the flask it will not suck back all the way into the reaction train. As pressure begins to
build it will pressure the liquid out of the suck back trap back into the scrubber trap.
Texium - 15-6-2015 at 15:58
@Magpie: That's a simple solution that should solve the problem and allow it to work properly. Thank you!
@BromicAcid: That also sounds like a good solution that would make my apparatus rather pointless. I'm still having a little bit of trouble picturing
how it works, but I'll try out that way next time I do an experiment with gases.
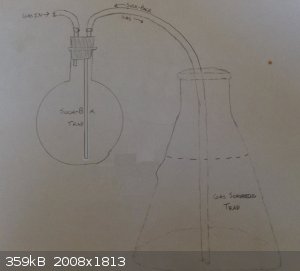
j_sum1 - 16-6-2015 at 06:34
Like this:
subsecret - 16-6-2015 at 07:40
Here's my design. The two parts are connected with a 45/50 joint.
[Edited on 16-6-2015 by subsecret]

kecskesajt - 17-6-2015 at 06:20
This might be helpful : http://m.youtube.com/watch?v=kqQSpRus-t0
