
nelsonB - 17-8-2015 at 09:56
Hi
so i remember have seen this before a long time ago
and i was wondering what was the reaction mechanism
under this
also would this be a better methode than using phosphorus trichloride that is expensive ?
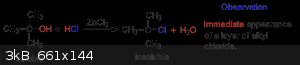
UC235 - 17-8-2015 at 10:09
A better method for what? Making methyl chloride? The combination of anhydrous ZnCl2 and conc. aq. HCl is called Lucas' reagent and is saturated with
ZnCl2. (something like 60g of ZnCl2 and 50g of conc. HCl is typical). Primary alcohols (methanol being the worst option) don't react readily. Tertiary
alcohols like the illustrated tert-butanol react immediately. The reaction mechanism is an SN1. On heating with primary alcohols or methanol, SN2 is
the likely mechanism.
gdflp - 17-8-2015 at 10:46
It's not necessary to use Lucas' reagent with tertiary alcohols. t-butanol readily reacts with concentrated hydrochloric acid at STP to form t-butyl
chloride. As Unintentional Chaos mentioned, the reaction follows an SN1 mechanism with secondary and tertiary alcohols, here's an example
with isopropanol.

nelsonB - 17-8-2015 at 12:08
UC235
so if tert-butanol react immediately
what would make it react faster,
i mean there a few difference and the boiling point is different
i guess its would be easier to extract and store the following chloride because its do not vaporise at room temp
