
ScienceHideout - 20-2-2016 at 15:41
Hi guys,
Long story but a LONG time ago I ran a reaction that failed. I ended up distilling phenol with a Hickman-type microscale distillation apparatuses. I
probably only have about a gram in it, so to me it is not worth saving. However, it is in one of those awful crystalline masses. For the longest time
I just had a cap on the distillation head, and I was keeping it. But today is the day when I need to finally get rid of it, and I am looking for a
good disposal technique. My ideal situation would be to:
a) DESTROY phenol, converting it into an innocuous substance
b) do this without sending any unconverted phenol down the drain
c) get NO phenol on me.
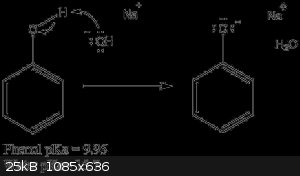
I was thinking to drop the still head into a 1L beaker full of 1M NaOH, leave it for an hour, and wash the liquid down the drain. This is the
procedure that is recommended in the Flinn handbook, because phenol is an acid. However, I am hesitant about this procedure because a) phenol is not
that soluble in water and b) I know nothing about sodium phenoxide safety.
If sodium phenoxide is pretty safe, I suppose another option is to pour some sort of alcohol into the Hickman head to dissolve it, then pour the
solution into NaOH. Perhaps if this is the way to go, a less volatile alcohol would be a good idea, like BuOH?
I am just curious what you guys think... if you were me, how would you get rid of phenol?
JJay - 20-2-2016 at 15:56
If I wanted to destroy some phenol, I would probably destroy it with sulfuric acid and a strong oxidizer (be careful doing this). You could also use
an incinerator. But if I had a gram, I'd probably just put it into a well-sealed vial with a label and store it.
UC235 - 20-2-2016 at 17:41
Just wash it down the drain. It has pretty reasonable water solubility (8% at 20C,). While it damages tissue when applied neat or in concentrated
solutions, it's really pretty harmless in dilute aq. solution. For example, Chloraseptic throat spray is 1.4% aq. Phenol meant to be sprayed into the
mouth. It's also readily biodegradable.
halogen - 20-2-2016 at 18:10
There is a reaction which phenoxide salt reacts with carbon dioxide to salicylic acid. Forget what it called. Who it's named after.
You could react it with formaldehyde and a catalyst to dispose of it. This forms, in addition to a minor amount of calixarenes, a hard insoluble
resin. This is the strategy employed for dispose of nuclear waste. Bury the lump.
woelen - 22-2-2016 at 07:30
You are talking about just 1 gram? If that is the case, I would rinse it away with a lot of water and flush the water with the phenol in it down the
drain. Adding some NaOH increases solubility and makes removal somewhat easier. The same is true for denatured ethanol.
Herr Haber - 22-2-2016 at 16:32
Melting point: 43c°
Use DHMO as a solvent 
Ozone - 22-2-2016 at 16:51
Just drop the whole piece in a bucket of water, wait a while, and carefully pour the whole thing down the drain.
O3

