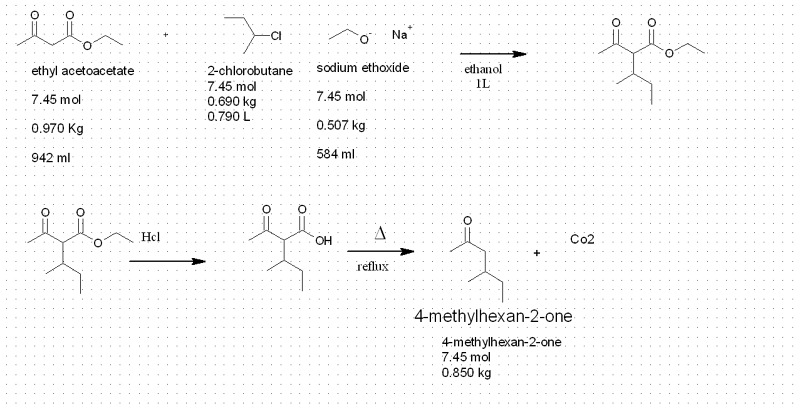
| I've never done any acetoacetic ester syntheses, but I have done a malonic ester synthesis (using a primary alkyl bromide, and anhydrous potassium
carbonate as a base). One cause of lower yield was formation of dialkylated product. However, this might be less likely to happen with a relatively
unreactive secondary alkyl chloride. |